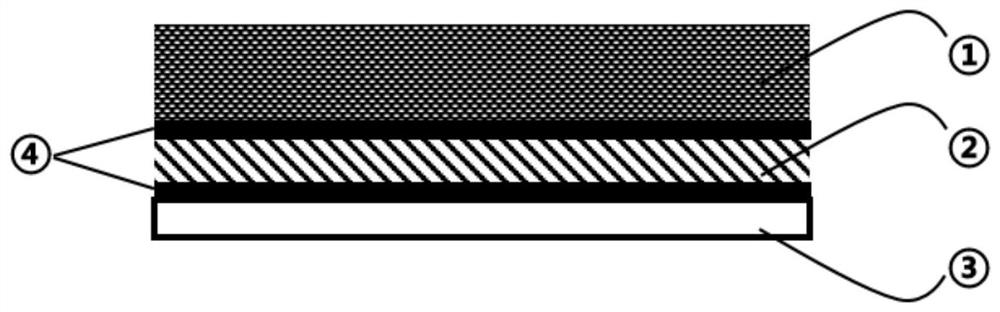
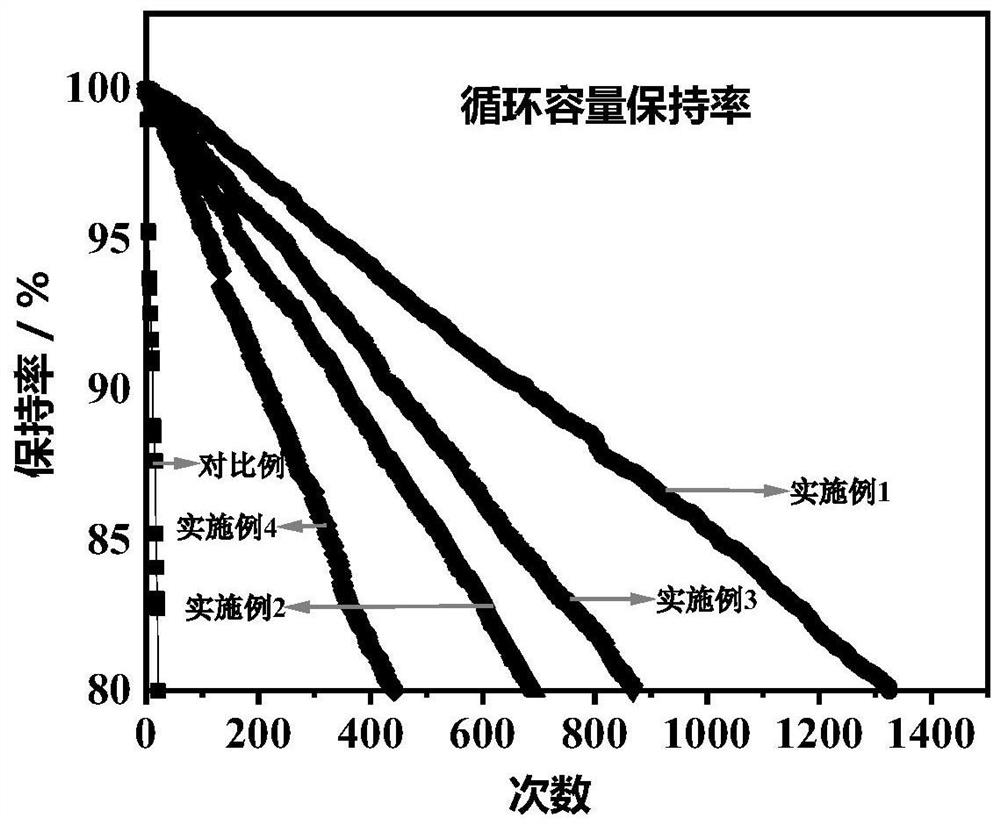
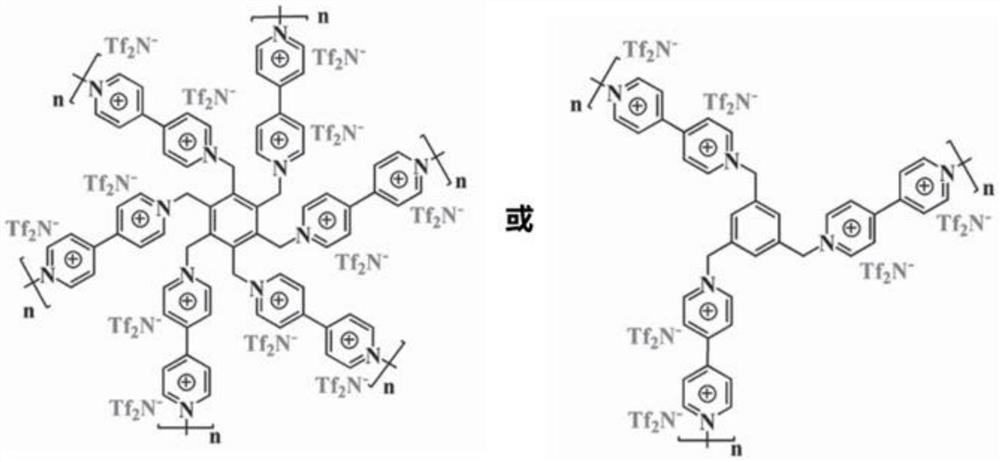
Solid-state electrolyte capable of in-situ polymerization and all-solid-state battery containing solid electrolyte
A solid-state electrolyte and high-quality technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems affecting the electrochemical performance of solid-state lithium-ion batteries, the reduction of active components in electrolytes, and the reduction of lithium-ion conductivity, etc., to improve the interface Contact site, improve electrochemical performance, reduce the effect of transmission impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also provides a method for preparing a precursor for the modification layer of the solid electrolyte, comprising the following steps:
[0050] S1. prepare prepolymer;
[0051] S2. Mixing the prepolymer, nitrile compound and lithium salt to obtain the precursor.
[0052] According to the present invention, step S1 specifically includes: prepolymerizing the bromomethylbenzene monomer and the pyridine monomer in a solvent system to obtain the prepolymer.
[0053] For example, the solvent can be NMP. Further, the prepolymerization reaction is carried out under an inert atmosphere. For example, under nitrogen or argon atmosphere.
[0054] According to the present invention, the temperature of the pre-polymerization is 100-140°C, exemplarily 100°C, 120°C, 140°C. Further, the time of the pre-polymerization is 18-24h, exemplarily 18h, 20h, 24h.
[0055] According to the present invention, step S1 further includes: performing solid-liquid separation on...
Embodiment 1
[0103] Preparation of PNCs-1:
[0104] (1) Dissolve 1.9g of hexa(bromomethyl)benzene and 1.64g of 4,4-bipyridine in 150mL of NMP solvent, and mix and stir for 2h under an argon atmosphere;
[0105] (2) Transfer the above-mentioned mixed materials to a polytetrafluoroethylene-lined autoclave, and heat at 120°C for 20h;
[0106] (3) The product is filtered, and washed with deionized water and ethanol more than 3 times;
[0107] (4) Dispersing the product in water, using 15wt% LiTFSI aqueous solution to carry out ion exchange 3 times, the time of each ion exchange reaction is 24h;
[0108] (5) Vacuum drying the product at 80°C to obtain precursor prepolymer powder;
[0109] (6) Add 1 g of PNCs-1 prepared in step (5) into 2 g of succinonitrile (SN), heat and stir at 80° C. for 1 h, and stir evenly;
[0110] (7) Add 0.15g LiTFSI to the above mixture, continue heating and stirring for 24h to obtain a viscous liquid, namely "PNCs-1".
[0111] Prepare the positive electrode materi...
Embodiment 2
[0115] Preparation of PNCs-2:
[0116] (1) Dissolve 1.9g 1,3,5-tris(bromomethyl)benzene and 1.64g 4,4-bipyridine in 150mL NMP solvent, mix and stir for 2h under argon atmosphere;
[0117] (2) Transfer the above-mentioned mixed materials to a polytetrafluoroethylene-lined autoclave, and heat at 120°C for 20h;
[0118] (3) The product is filtered, and washed with deionized water and ethanol more than 3 times;
[0119] (4) Dispersing the product in water, using 15wt% LiTFSI aqueous solution to carry out ion exchange 3 times, the time of each ion exchange reaction is 24h;
[0120] (5) Vacuum drying the product at 80°C to obtain precursor prepolymer powder;
[0121] (6) Add 1 g of PNCs-1 prepared in step (5) into 2 g of succinonitrile (SN), heat and stir at 80° C. for 1 h, and stir evenly;
[0122] (7) Add 0.15g LiTFSI to the above mixture, continue heating and stirring for 24h to obtain a viscous liquid, namely "PNCs-2".
[0123] Prepare the positive electrode material: use ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| peel strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com