Method for preparing rare earth oxyfluoride
A technology of oxyfluoride and rare earth, which is applied in the field of preparation of rare earth oxyfluoride, can solve the problems of high acidity, difficult treatment, and high fluoride ion concentration of fluorine-containing waste liquid, and achieve effective utilization, high recovery rate, and simple process flow Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
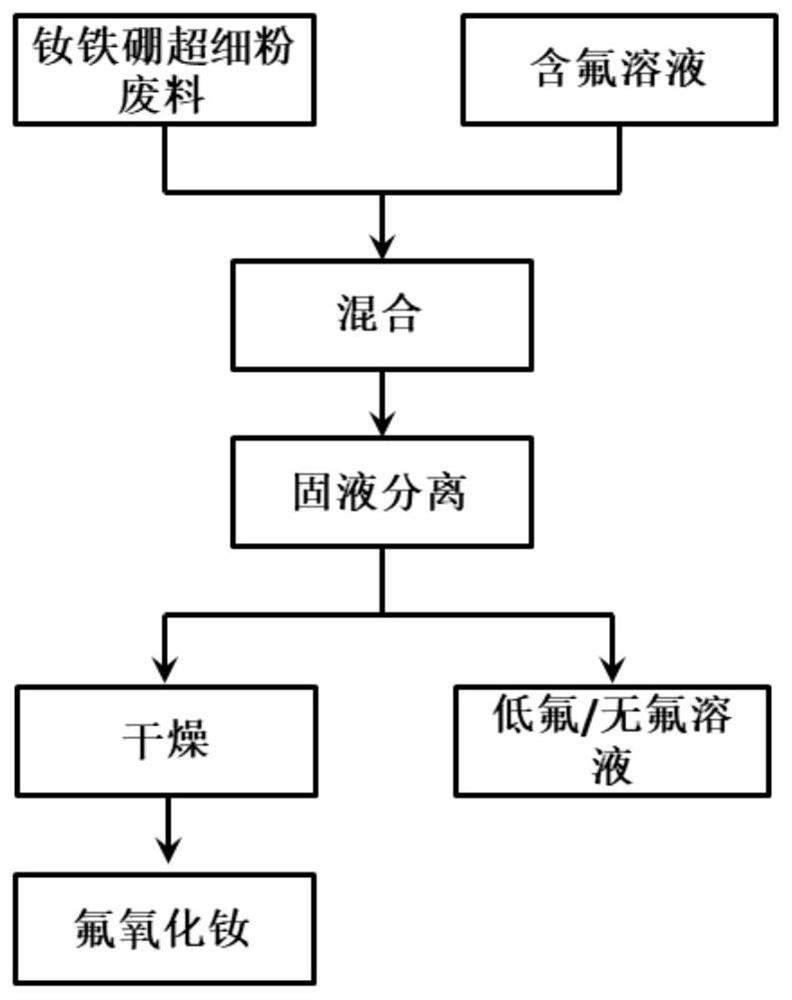
[0044] This embodiment provides a method for preparing rare earth oxyfluoride, comprising the following steps:
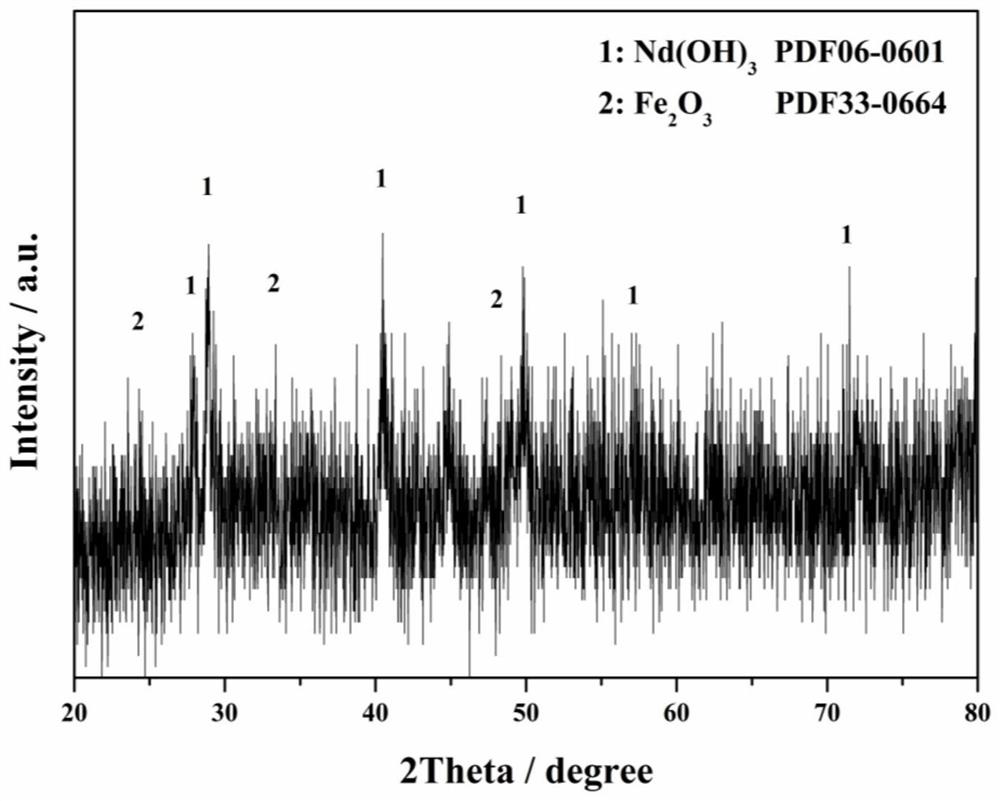
[0045] (1) Mix NdFeB superfine powder waste with a solution containing fluoride ions, stir and react at room temperature for 12 hours to completely dissolve iron oxide, and Nd(OH) 3 React with fluoride ions to form Nd(OH) 2 F precipitation;
[0046] (2) precipitation in the centrifugal separation reaction system;
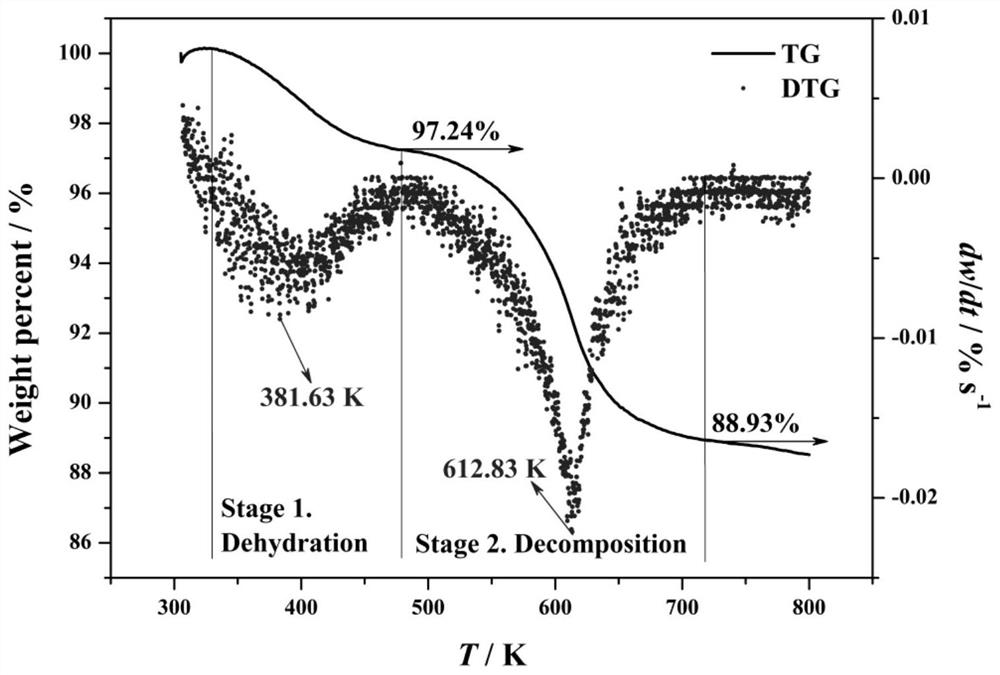
[0047] (3) Dry the separated precipitate at 650° C. for 4 hours to obtain NdOF powder.
[0048] Among them, the solution containing fluoride ion has a fluoride ion concentration of 0.1mol L -1 Ferric fluoride and a concentration of 1mol L -1The nitric acid solution has a pH value of 0.
[0049] After detection and calculation, the fluoride ion concentration in the solution after separation and precipitation is 0.005mol L -1 , the pH value is 0.51; the total recovery rate of rare earth is 99.5%.
[0050] Figure 5 For the SEM images of different ma...
Embodiment 2
[0052] This embodiment provides a method for preparing rare earth oxyfluoride, comprising the following steps:
[0053] (1) Mix the NdFeB ultrafine powder waste with the solution containing fluoride ions, stir and react at room temperature for 8 hours, so that the iron oxide is completely dissolved, and the Nd(OH) 3 React with fluoride ions to form Nd(OH) 2 F precipitation;
[0054] (2) precipitation in the centrifugal separation reaction system;
[0055] (3) Dry the separated precipitate at 110° C. for 24 hours, and then at 600° C. for 2 hours to obtain NdOF powder.
[0056] Among them, the solution containing fluoride ion has a fluoride ion concentration of 15mol L -1 Potassium fluoride and nitric acid solution, the pH of the solution is 1.
[0057] After detection and calculation, the fluoride ion concentration of the solution after separation and precipitation is 14.98mol L -1 , the pH value is 7.2; the total recovery rate of rare earth is 98.8%.
Embodiment 3
[0059] This embodiment provides a method for preparing rare earth oxyfluoride, comprising the following steps:
[0060] (1) Mix NdFeB superfine powder waste with a solution containing fluoride ions, stir and react at room temperature for 12 hours to completely dissolve iron oxide, and Nd(OH) 3 React with fluoride ions to form Nd(OH) 2 F precipitation;
[0061] (2) precipitation in the centrifugal separation reaction system;
[0062] (3) Dry the separated precipitate at 110° C. for 24 hours, and then at 600° C. for 2 hours to obtain NdOF powder.
[0063] Among them, the solution containing fluoride ion has a fluoride ion concentration of 15mol L -1 Potassium fluoride and nitric acid solution, the pH of the solution is 3.
[0064] After detection and calculation, the fluoride ion concentration in the solution after separation and precipitation is 14.98mol L -1 , the pH value is 7.1; the total recovery rate of rare earth is 95.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com