Pharmaceutical application of combination of salvianolic acid A, salvianolic acid B and salvianolic acid C for resisting 2019-nCov virus and medicine
A 2019-ncov, salvianolic acid technology, applied in the field of antiviral drug preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
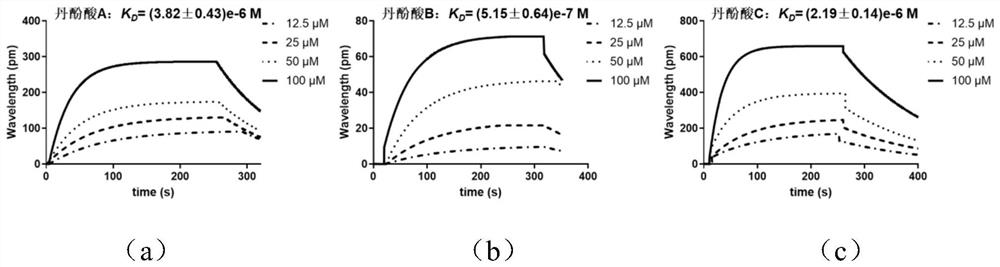
[0025] Embodiment 1, SPR method detects the binding ability of salvianolic acid A, salvianolic acid B and salvianolic acid C and ACE2 protein and S protein
[0026] Experimental materials: ACE2 protein (purchased from Beijing Yiqiao), salvianolic acid A (purchased from Baoji Chenguang), salvianolic acid B (purchased from Shanghai Anpu) and salvianolic acid C (purchased from Baoji Chenguang), SPR, COOH Chips and corresponding activation reagents (purchased from Nicoya, Canada).
[0027] Preparation method: install a carboxyl chip in the Open SPR instrument, and pump the running buffer at the maximum flow rate (150 μL / min) to make it full of the detection cell. After reaching the signal baseline, inject 80% isopropanol to degas and clean the chip surface with 10 mM HCl. After reaching the signal baseline again, adjust the flow rate to 20 μL / min. Immediately mix an equal amount of coupling reagent (EDC / NHS) and inject it into the injection valve, activate the chip for 5 minutes...
Embodiment 2
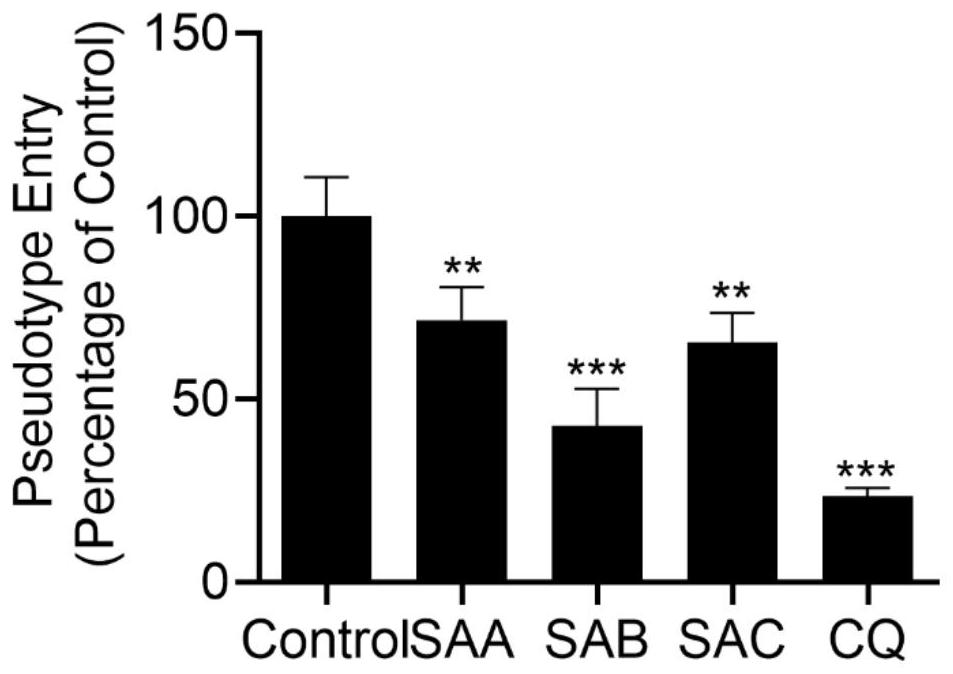
[0030] Example 2. Salvianolic acid A, salvianolic acid B and salvianolic acid C inhibit 2019-nCov pseudovirus from infecting cells with high expression of ACE2.
[0031] ACE2 high-expressing cells were seeded in 96-well plates, and each well was supplemented with medium to 50 μL. 37°C, 5% CO 2 Incubate in the incubator for 2 hours to adhere to the wall. Add 20 μM salvianolic acid A, salvianolic acid B or salvianolic acid C prepared in culture medium respectively, add 5uL 2019-CoV-2 Spike pseudovirus to each well for 4 hours, make up to 100 μL culture volume, continue infection for 6-8 hours, replace Make 200 μL of new complete medium and continue culturing at 37°C for 48h. The Luciferase Assay System kit detects the luminescence value of Luciferase.
[0032] see image 3 , after administration, the luminous intensity of Luciferase decreased compared with the control group. Chloroquine (20 μM) was used as an antiviral positive control. From image 3 It can be seen from t...
Embodiment 3
[0033] Example 3 Combined administration of salvianolic acid A, salvianolic acid B and salvianolic acid C inhibits 2019-nCov pseudovirus from infecting cells with high expression of ACE2.
[0034] ACE2 high-expressing cells were seeded in 96-well plates, and each well was supplemented with medium to 50 μL. 37°C, 5% CO 2 Incubate in the incubator for 2 hours to adhere to the wall. 10 μM salvianolic acid B or 2.5 μM salvianolic acid A, 5 μM salvianolic acid B and 2.5 μM salvianolic acid C were administered alone, and incubated at 37°C for 2 hours. Add 5uL 2019-CoV-2 Spike pseudovirus to each well to infect for 4 hours, make up to 100 μL culture volume, continue infection for 6-8 hours, replace with 200 μL new complete medium, and continue to culture at 37°C for 48 hours. The Luciferase AssaySystem kit detects the luminescence value of Luciferase.
[0035] see Figure 4 , after co-administration, the luminous intensity of Luciferase decreased compared with the control group. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com