Pharmaceutical co-crystal of elagolix and palmoxiric acid and preparation method of pharmaceutical co-crystal
A technology of pamoic acid and co-crystal, which is applied in the field of drug co-crystal of elagolix and pamoic acid and its preparation, and can solve the problems of low bioavailability and low solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 Preparation of elagolix drug cocrystal
[0050] In a common 50ml three-neck flask, add 50mg of elagoli (free acid) and 24.5mg of pamoic acid, then add 2ml of methanol, heat up and reflux for 30min, then slowly cool down, and the mixed solution slowly volatilizes at room temperature for about 1 week, and obtain White powdery solid, HPLC purity test is 99.3%.
Embodiment 2
[0051] Example 2 PXRD detection of elagolix drug cocrystal
[0052] 1. Sample
[0053] The solid obtained by the method of Example 1 was ground and sieved and accurately weighed, and a sample of 50 mg was detected.
[0054] 2. PXRD detection
[0055] The D8 X-ray powder diffractometer of German Bruker Company was used for PXRD detection of drug cocrystals.
[0056] Experimental conditions:
[0057]
[0058] Pipe pressure 40kV;
[0059] Pipe flow 40mA;
[0060] Time constant 0.1S;
[0061] The test range is 5°~90°.
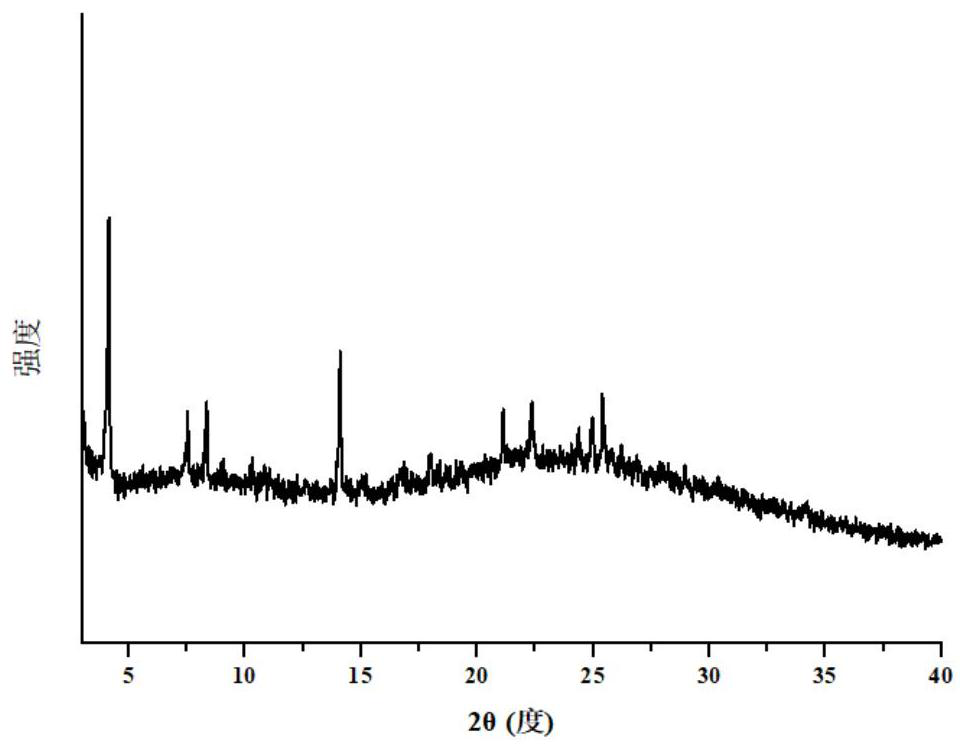
[0062] 3. Results
[0063] The results of X-ray powder diffraction of elagoli drug cocrystal are shown in Table 1 and figure 1 shown.
[0064] Table 1 PXRD peak list of elagolix drug cocrystal
[0065] 2θ d BG Height % area % wxya 4.135 21.352 372 776 100 5111 100 0.112 7.529 11.7325 353 210 27.1 2884 56.4 0.234 8.351 10.5787 359 235 30.3 1543 30.2 0.112 9.08 9.7311 346 75 9.7 531 10.4 0...
Embodiment 3
[0066] The thermogravimetric analysis of embodiment 3 elagolix drug cocrystals
[0067] 1. Sample
[0068] Weigh 5-10 mg of the solid obtained by the method of Example 1 of the sample for detection.
[0069] 2. Thermogravimetric analysis
[0070] Mettler Thermogravimetric Analyzer (TGA-1) was used to detect the out-of-order of the drug.
[0071] Experimental conditions:
[0073] The temperature detection range is 30~400℃;
[0074] Heating rate 10K / min;
[0075] The nitrogen flow rate was 50ml / min.
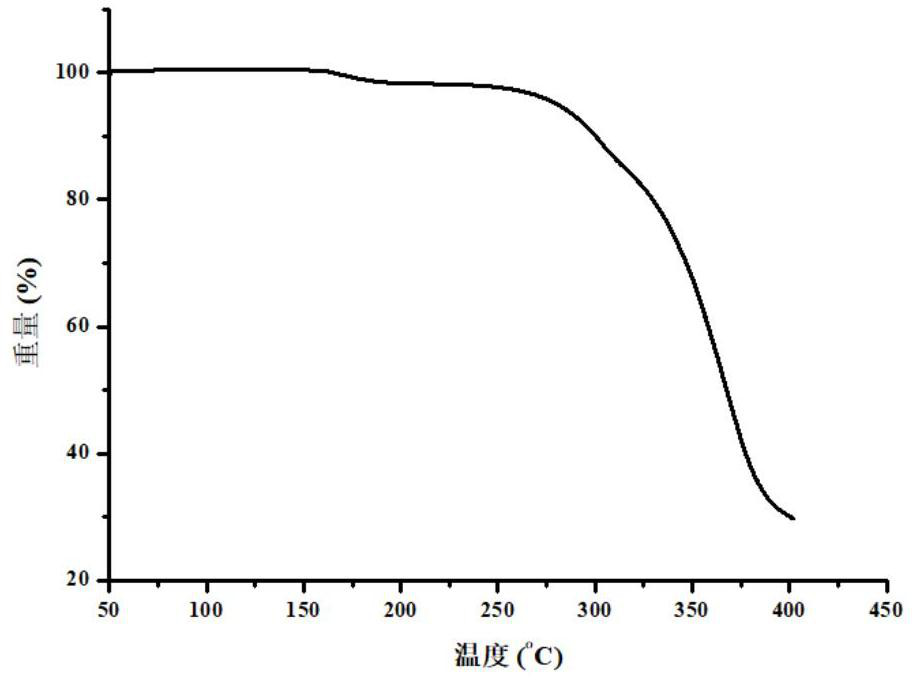
[0076] 3. Results
[0077] The result is as figure 2 As shown, the drug eutectic has no obvious out-of-order phenomenon in the heating stage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com