Water-based in-situ gel ophthalmic preparation for treating xerophthalmia
An in-situ gel and ophthalmic preparation technology, applied in the field of medicine, can solve the problem of not specifying thickeners and the like, and achieve the effects of reducing the frequency of frequent medication, reducing systemic absorption, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
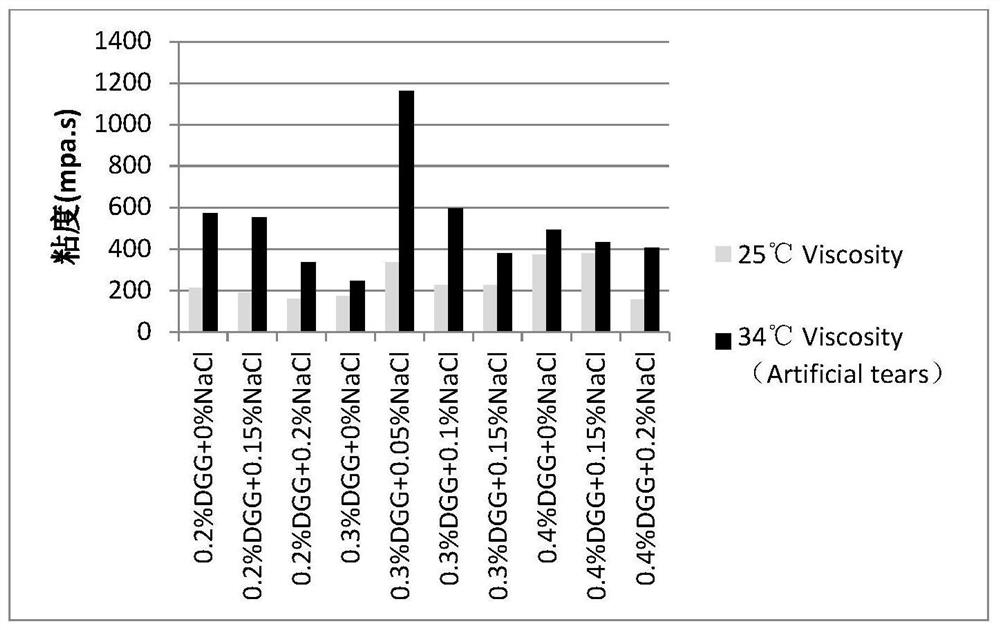
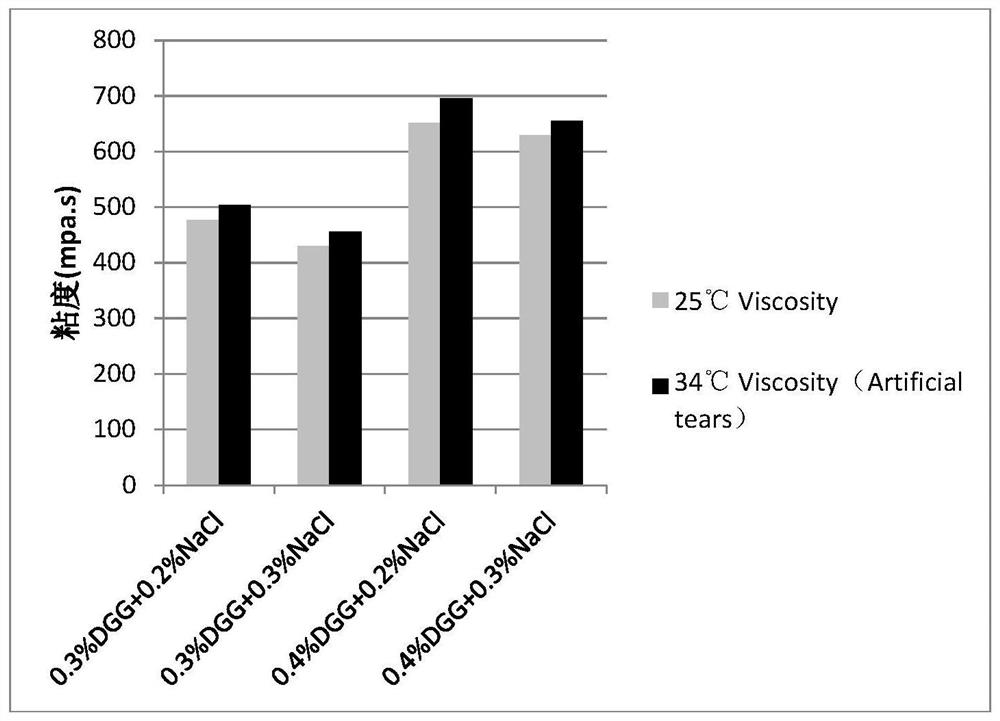
[0045] Embodiment 1: the mensuration of gel matrix concentration
[0046] The gel matrix solution samples containing 0.1% diclofenac sodium and 0.5% sodium hyaluronate at different concentrations are shown in Table 2-5:
[0047] Table 2: Concentration of Gel Matrix DGG
[0048]
[0049]
[0050] Table 3: Concentration of Xanthan Gum in Gel Matrix
[0051]
[0052] Table 4: Gel Matrix Carrageenan Concentrations
[0053]
[0054] Table 5: Concentration of Sodium Alginate in Gel Matrix
[0055]
[0056] Preparation method of gel solution
[0057] Accurately weigh a certain amount of sodium chloride, and slowly add 85 grams of deionized water. Stir until the sodium chloride is completely dissolved, and slowly add the gel base and sodium hyaluronate to the above mixture with continuous stirring. The solution was placed in a 90°C water bath and stirred for 1 hour. The mixture was then cooled to room temperature. Weigh 0.1 g of diclofenac sodium, and slowly add ...
Embodiment 2
[0073] Embodiment 2: the aqueous in situ gel ophthalmic preparation of the present embodiment
[0074] The instant ophthalmic gel containing 0.1% diclofenac sodium and 0.5% sodium hyaluronate, the specific prescription is as follows:
[0075] Diclofenac Sodium 0.1wt%, Sodium Hyaluronate 0.5%wt%, Deacetylated Gellan Gum 0.4wt%, Polyoxyethylene 35 Castor Oil 5.0wt%, Vitamin E Macrogol Succinate 0.5%, Mannitol 4.0wt% %, 0.02wt% butyl p-hydroxybenzoate, add an appropriate amount of tromethamine hydrochloride buffer solution, add water for injection to 100g, and make 100g specifications of ophthalmic instant gel containing 0.1% diclofenac sodium and 0.5% sodium hyaluronate glue. (Table 10)
[0076]
[0077] In Table 10, Sample 1 is a formulation in which a combination of polyoxyethylene 35 castor oil and vitamin E polyethylene glycol succinate was added as a solubilizer. Sample 2 is a formulation with pH adjusted to a strongly alkaline solution (pH 8.5) without a solubilizer,...
Embodiment 3
[0106] Example 3: Aqueous in situ gel ophthalmic formulations composed of different pH
[0107] Different pH in situ gel solutions containing 0.1% diclofenac sodium and 0.5% sodium hyaluronate, specific prescriptions are as follows:
[0108] Diclofenac Sodium 0.1wt%, Sodium Hyaluronate 0.5%wt%, Deacetylated Gellan Gum 0.4wt%, Polyoxyethylene 35 Castor Oil 5.0wt%, Vitamin E Macrogol Succinate 0.5%, Mannitol 4.0wt% , butyl p-hydroxybenzoate 0.02wt%, add an appropriate amount of tromethamine hydrochloride buffer or hydrochloric acid, and water for injection, make 100g ophthalmic in situ gel containing 0.1% diclofenac sodium and 0.5% sodium hyaluronate ( Table 16).
[0109]
[0110] Stability Study: Samples 1 / 2 / 3 / 4 were prepared and divided into multi-dose eye drop bottles and stored in a stability room at 25°C. Samples were taken on days 0, 3, 6, and 10, respectively.
[0111] Features: traits, pH, content of diclofenac sodium.
[0112] Table 17: Stability test results for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com