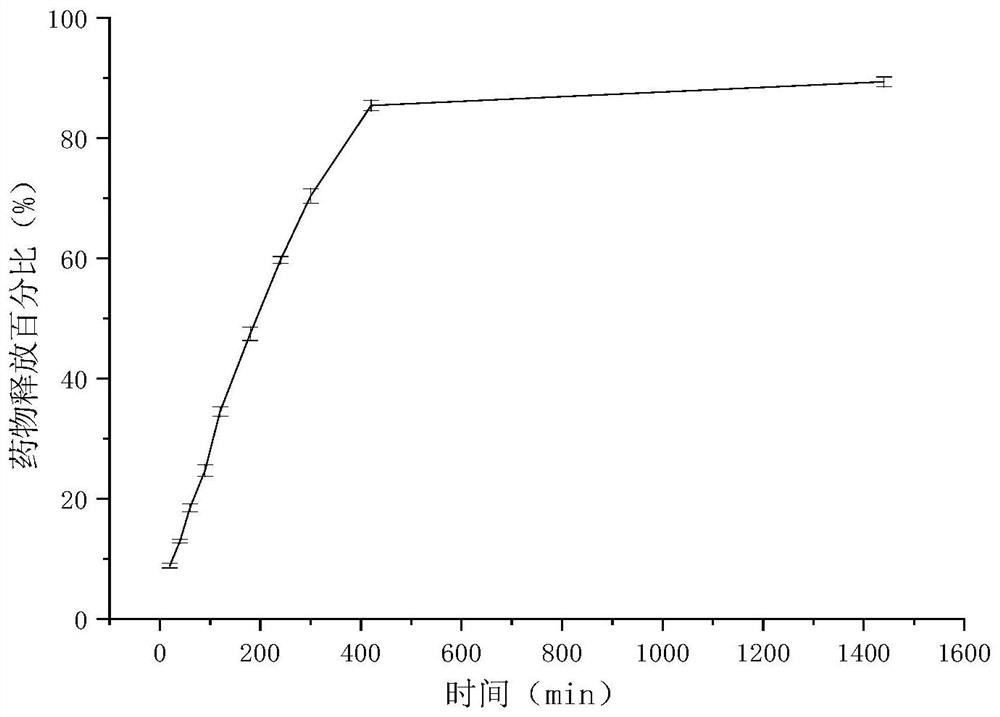
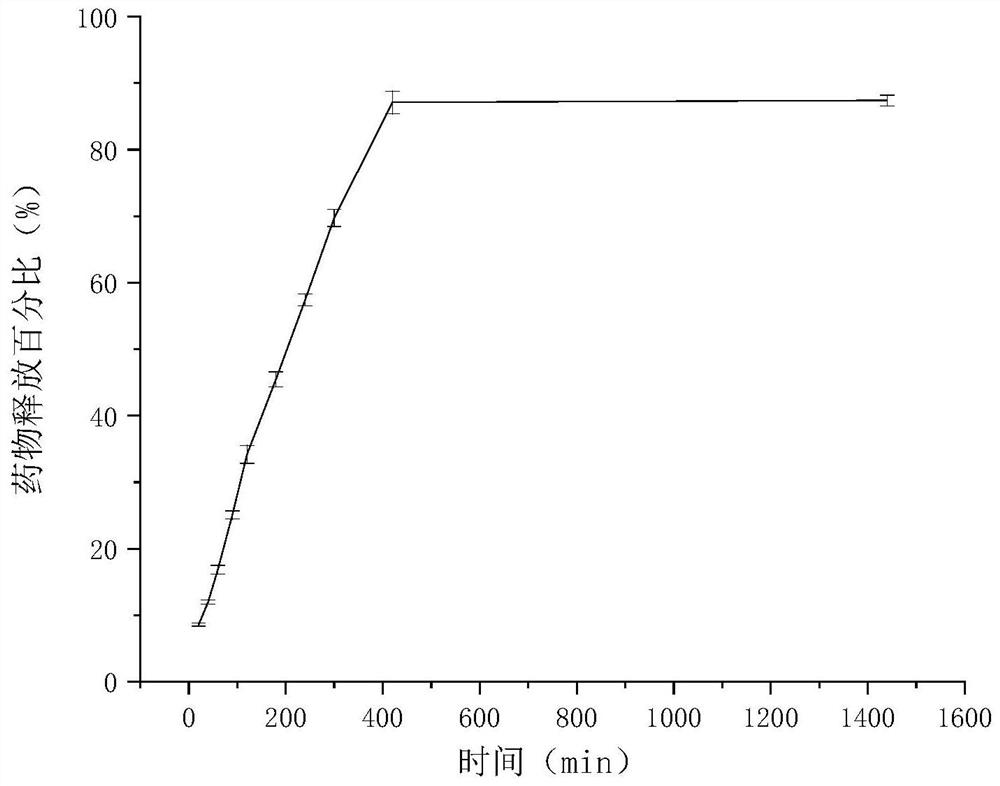
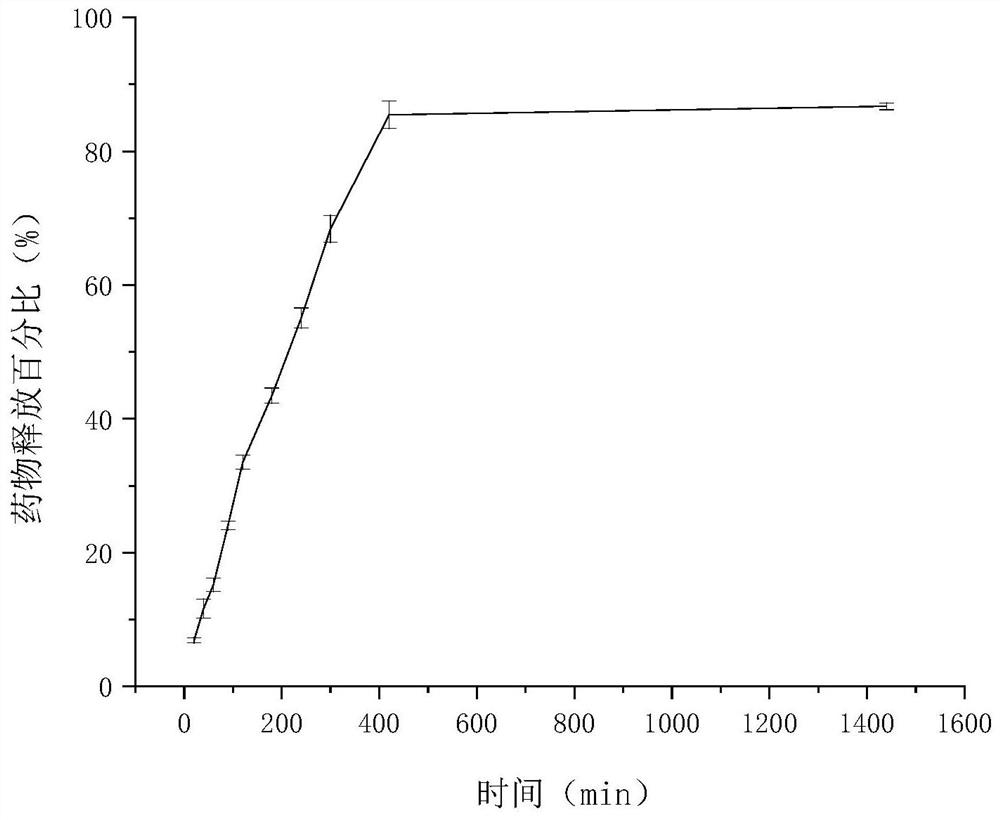
Clonidine hydrochloride sustained-release micro-tablets as well as preparation method and application thereof
A clonidine hydrochloride, sustained-release technology, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem that the treatment effect of eye diseases is not very satisfactory, and the drug release speed is not easy to obtain. control, systemic arterial pressure reduction and other issues, to achieve good practical application value, good controlled release effect, and the effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In view of this, the present invention provides an ophthalmic clonidine hydrochloride sustained-release microtablet and its preparation method and application.
[0034] In one embodiment of the present invention, a kind of clonidine hydrochloride slow-release microtablet is provided, and by weight percentage, it is composed of:
[0035] Clonidine hydrochloride: 3-6%
[0036] Gel skeleton: 68-86%
[0037] Lubricant: 0.5~2%
[0038] Filling agent: 10-25%.
[0039] In a specific embodiment, the gel skeleton is selected from one or more of hypromellose, carbomer, ethyl cellulose, more preferably hypromellose and carbomer Further, the weight ratio of hypromellose and carbomer is: 65-80:3-6.
[0040] Hypromellose and carbomer are used as the hydrophilic gel skeleton, and the two cooperate with each other to play the role of slow-release medicine. After the inventor's experimental research, the amount of hypromellose and carbomer used in the embodiment of the present inven...
Embodiment 1
[0050] The prescription of clonidine hydrochloride sustained-release microtablets is:
[0051] Clonidine hydrochloride: 5%
[0052] Hypromellose RG4T: 75%
[0053] Carbomer 974P: 4%
[0054] Sodium stearyl fumarate: 1%
[0055] Mannitol: 15%
[0056] The above-mentioned raw materials were sterilized by γ-ray irradiation before use, and the following operations were all carried out in a sterile environment. The raw materials were mixed evenly with a mortar, pulverized, and pressed into small tablets with a diameter of 3 mm by using a direct compression method using a heterogeneous tablet press ((Korsch-EKO model, Berlin, Germany) with maximum pressure.
Embodiment 2
[0058] The prescription of clonidine hydrochloride sustained-release microtablets is:
[0059] Clonidine hydrochloride: 5%
[0060] Hypromellose RG4T: 65%
[0061] Carbomer 974P: 10%
[0062] Sodium stearyl fumarate: 1%
[0063] Mannitol: 17%.
[0064] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com