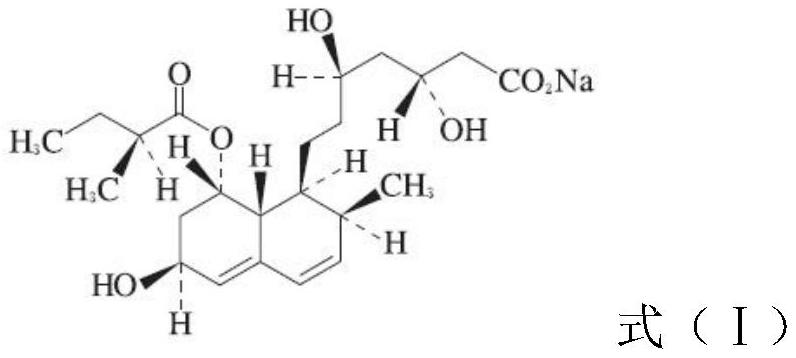
Pravastatin sodium enteric-coated tablet and preparation method thereof
A technology of pravastatin sodium and enteric-coated tablets, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, coatings, etc., which can solve the problem of poor fluidity and compressibility of raw materials, reduction of adverse reactions, and difficulty in meeting direct compression requirements Process requirements and other issues to achieve the effect of improving poor fluidity, high bioavailability, and uniform release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 5
[0043] Embodiment 5 stability test
[0044] Take embodiment 1 sample as investigation object, investigate the stability of accelerated test, result is as shown in table 2:
[0045] Table 2
[0046]
Embodiment 6
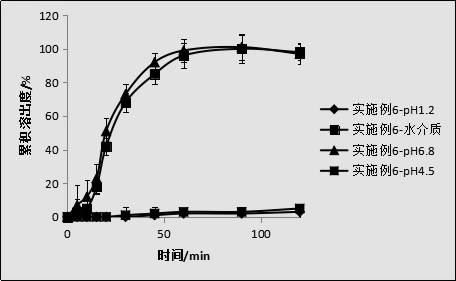
[0048] Embodiment 6 dissolution test
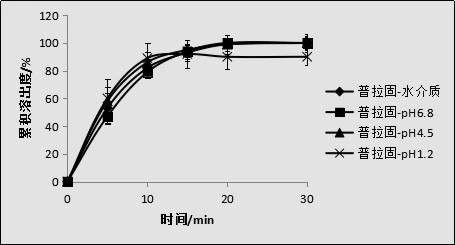
[0049] Taking the sample of Example 1 as the object of investigation, investigate the dissolution curves of the gained pravastatin sodium enteric-coated tablets of the present invention and commercially available pravastatin sodium tablets (trade name: Pravastatin), the results are shown in figure 1 .
Embodiment 7
[0050] Embodiment 7 pharmacokinetic test
[0051] Take embodiment 1 sample as object of investigation, investigate gained pravastatin sodium enteric-coated tablet of the present invention and commercially available pravastatin sodium tablet (trade name: pravastatin) pharmacokinetic test, the results are as follows:
[0052] Test preparation: Pravastatin sodium enteric-coated tablets obtained in Example 1 (specification: 10 mg / tablet);
[0053] Reference drug: purchase Sino-US Shanghai Bristol-Myers Squibb Pharmaceutical Co., Ltd. (Pulagu, specification: 10mg / tablet);
[0054] Select 20 healthy male subjects, aged (23.2±1.2) years old, body mass (65.3±5.2) kg, height (172.3±5.2) cm, all subjects were asked about medical history and physical examination before the experiment, electrocardiogram , chest X-ray, liver function, kidney function, blood routine, urine routine examination and mental state were all normal. The subjects did not take other drugs 2 weeks before and during...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com