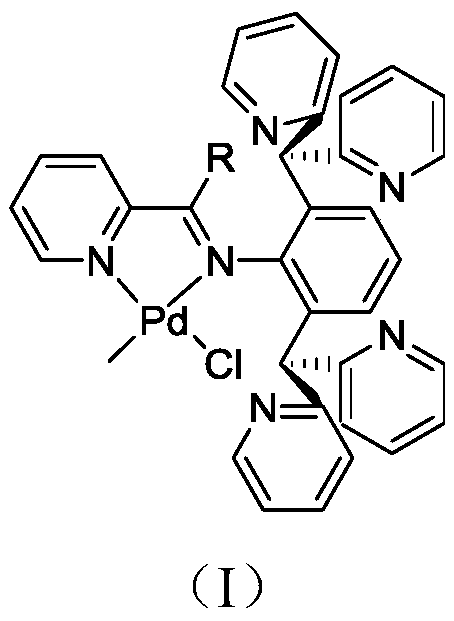
Complex for catalyzing polymerization of 4-methyl-1-pentene and preparation method thereof
A complex, methyl technology, applied in the direction of palladium organic compounds, platinum group organic compounds, chemical instruments and methods, etc., can solve the problem of inability to catalyze the copolymerization of olefins and polar monomers, the expensive consumption of cocatalyst methylaluminoxane, It is difficult to control the shape and particle size of the polymer to achieve the effects of increasing instability, enhancing electrophilicity, and inhibiting rotation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
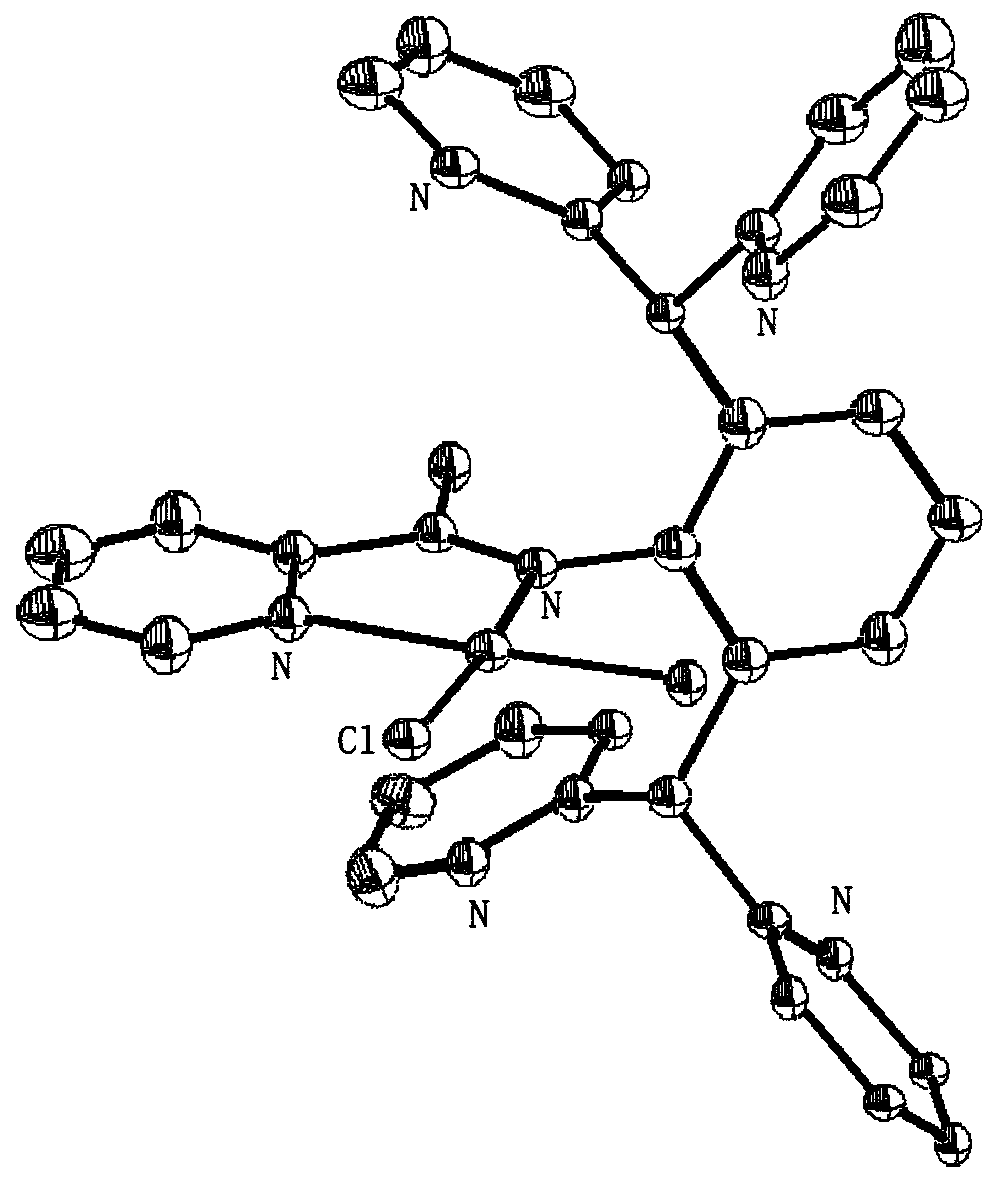
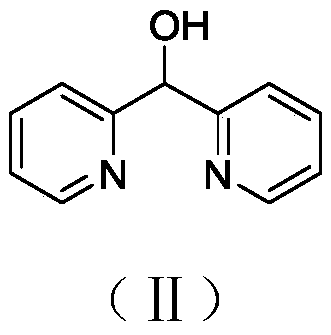
[0038] This embodiment provides a kind of bis(pyridin-2-yl)methanol, and its synthesis method is as follows.
[0039] Synthesis of bis(pyridin-2-yl)methanol: set the temperature of the thermostatic freezer at -78°C, and when the temperature reached -78°C, add 60mL of tetrahydrofuran and 2-bromopyridine (1.9mL, 20.0mmol) into the branch Slowly add n-butyllithium (2.5M dissolved in n-hexane, 8.0mL) dropwise into the flask. The reaction was stirred at -78°C for about 15 minutes, then 2-pyridinecarbaldehyde (1.9 mL, 20.0 mmol) was added dropwise. The reaction mixture was stirred at -78 °C for 30 min, then at room temperature for 2 h, then washed with saturated NH 4 Aqueous Cl solution quenched the reaction. After diluting the reaction mixture with ethyl acetate, the organic layer was separated and extracted twice with ethyl acetate. The combined organic fractions were washed with brine, dried (MgSO4), filtered and concentrated in vacuo to give a yellow liquid in 65.7% yield. 1...
Embodiment 2
[0041] This embodiment provides a kind of 2,6-bis(bis(pyridin-2-yl)methyl)aniline, and its synthesis method is as follows.
[0042] Synthesis of 2,6-bis(bis(pyridin-2-yl)methyl)aniline: Weigh aniline (2.19g, 23.5mmol) and bis(pyridin-2-yl)methanol (8.75g, 47mmol) into 150mL In the branch bottle, install the reflux condenser, heat (about 80°C), and weigh the ZnCl 2 (3g, 23.5mmol) was dissolved with concentrated HCl, and after p-toluidine and benzhydryl were melted, ZnCl was added dropwise 2 ·The HCl mixed solution was heated up to 170°C and reacted for 4 hours. After the reaction stopped, cool to room temperature and add CH 2 Cl 2 Dissolve the solid, extract, save the organic phase, add Na 2 CO 3 Remove HCl, filter, retain the filtrate, add silica gel to the filtrate to absorb impurities, filter, spin the filtrate to obtain a solid powder, wash with ethyl acetate to obtain a white powder, and the yield is 76.2%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):8.45(d,4H,Py),7.60(t,4H,Py...
Embodiment 3
[0044] This example provides a pyridine-imine ligand L1, the synthesis method of which is as follows.
[0045]Synthesis of pyridine-imine ligand L1: Weigh pyridine-2-carbaldehyde (0.536g, 5.0mmol), 2,6-bis(bis(pyridin-2-yl)methyl)aniline (2.15g, 5.0mmol) An appropriate amount of p-toluenesulfonic acid was dissolved in 80 mL of toluene, refluxed at 130°C overnight, and the crude product was recrystallized with ethanol to obtain yellow crystals after the solvent was spin-dried, with a yield of 80.8%. 1 H-NMR (400MHz, CDCl 3 ),δ(ppm):8.56-8.45(d,5H,Py),8.50(s,1H,CH),7.85-7.81(d,2H,Py),7.60(t,4H,Py),7.46-7.36 (t,6H,Py),7.11(t,4H,Py),5.34(s,2H,CH). 13 C-NMR (100MHz, CDCl 3 ), δ(ppm): 157.81, 151.70, 145.76, 136.89, 136.05, 131.16, 127.20, 126.36, 126.02, 125.75, 124.29, 120.50, 40.27.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com