Application of kalopanax saponin A in preparation of anti-candida albicans drugs, drug preparation of kalopanax saponin A, and drug preparation for diseases caused by candida albicans
A technology of Candida albicans and thorny saponins, applied in the field of biomedicine, can solve the problems of high toxicity and side effects, easy drug resistance, etc., and achieve the effects of good virulence, reduced virulence, and less drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
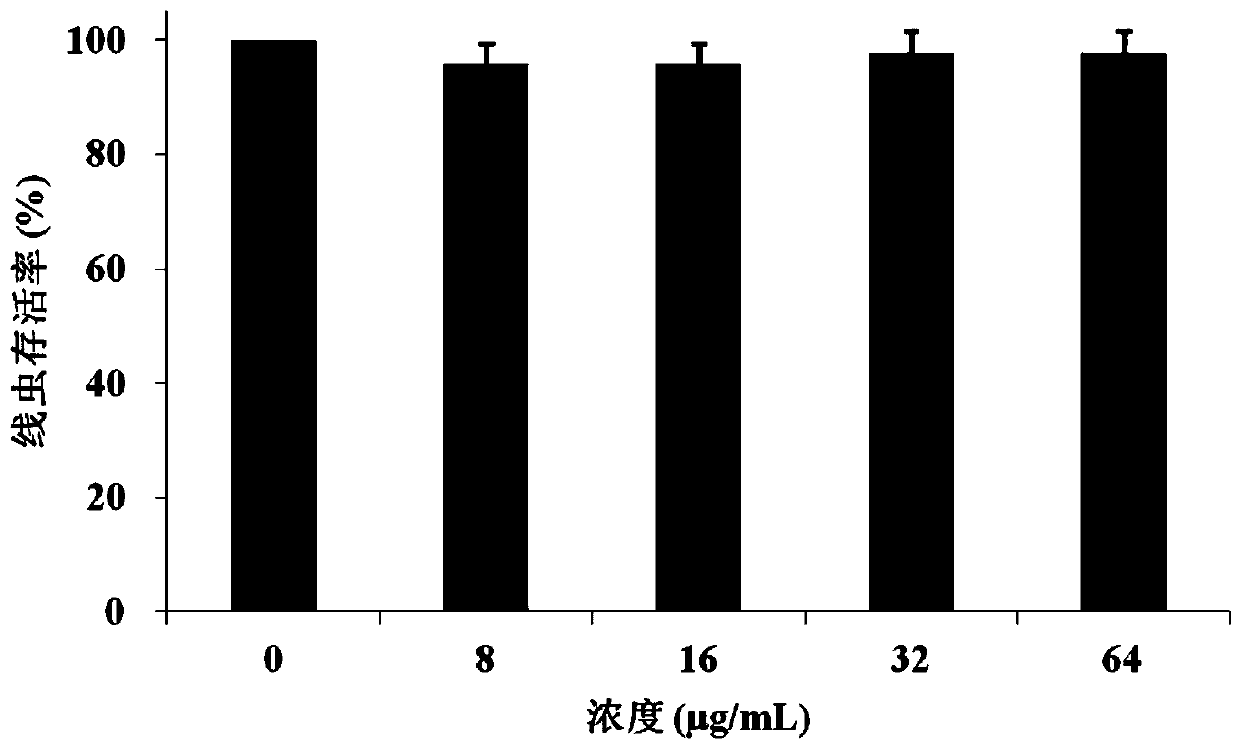
[0023] Embodiment 1Effect of thorny barley saponin A on the cytotoxicity of Caenorhabditis elegans
[0024] 1. Test drug
[0025] Robinia saponin A: It is extracted from the plant Robinia by the laboratory of Xuzhou Medical University, and its purity is greater than 98% as detected by analytical liquid phase.
[0026] Amphotericin B: American Sigma Reagent Company.
[0027] Dimethyl sulfoxide: American Sigma Reagent Company.
[0028] Accurately weigh the thorny bark saponin A and amphotericin B, use dimethyl sulfoxide (DMSO) as a solvent to prepare a mother solution with a final concentration of 10 mg / mL, and store it at -20°C. During the experiment, it was ensured that the content of dimethyl sulfoxide in all treatment groups was not higher than 1%.
[0029] 2. Preparation of C. elegans
[0030] Wild-type Caenorhabditis elegans N2 (purchased from the American Nematode Genetics Center) was used to carry out the toxicity evaluation experiment of apricoside A. Inoculate the...
Embodiment 2
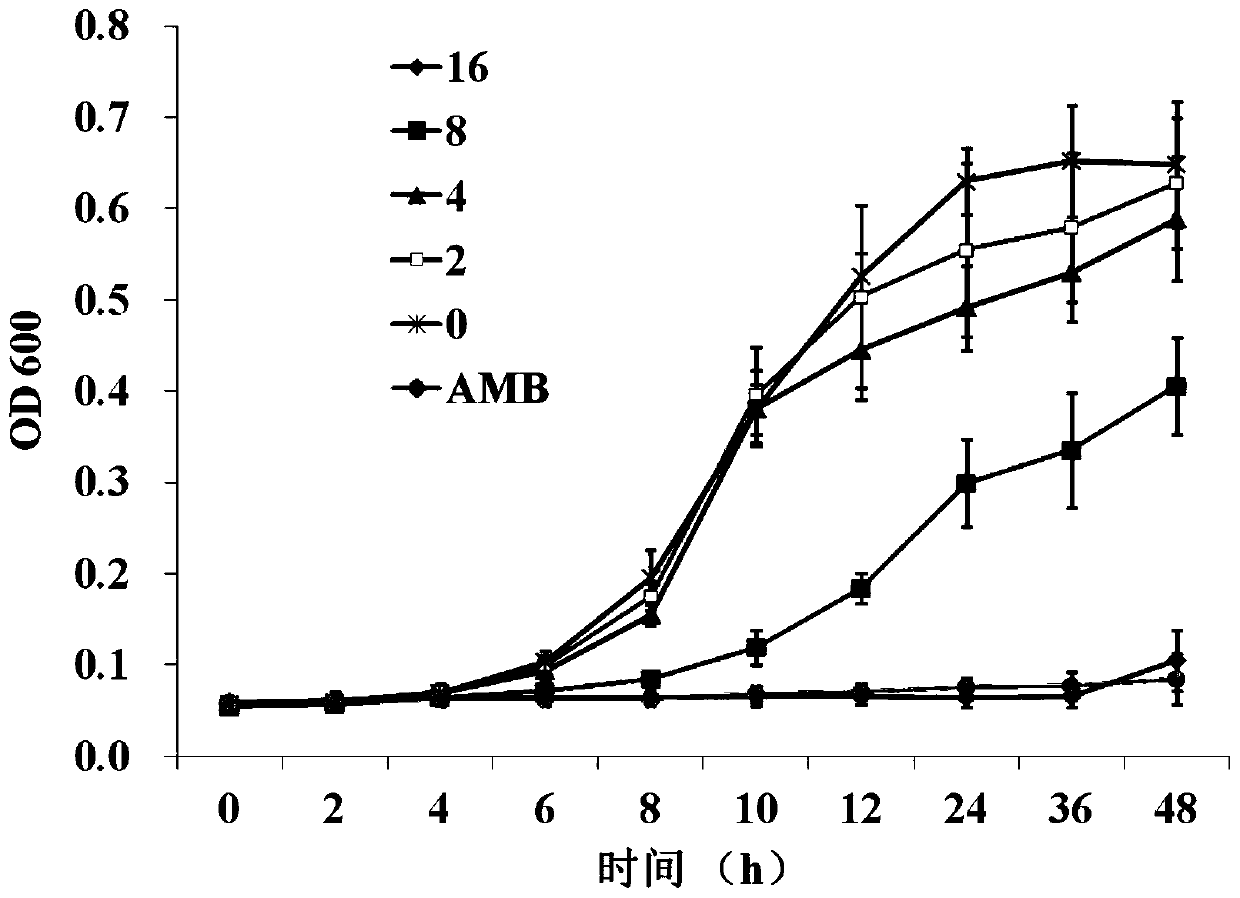
[0034] Embodiment 2Effects of Echinopsis saponin A on the proliferation of Candida albicans
[0035] 1. Preparation of Experimental Candida Strains
[0036] Candida albicans wild-type strain YEM30, clinical isolates of Candida tropicalis CT171221301, Candida glabrata CG171122302, and Candida parapsilosis CP18092240 were all isolated from clinical samples submitted for inspection by the Laboratory Department of the Affiliated Hospital of Xuzhou Medical University, and were identified and confirmed by instruments and chromogenic medium. Clinically isolated azole multidrug-resistant strains CA10 and CA148 were donated by the Laboratory Department of Qianfoshan Hospital.
[0037] The above bacterial strains are suspended in seed preservation solution (physiological saline containing 20% glycerol), and stored at -80°C for a long time. Before the experiment, the bacterial strain was transferred to YPD solid medium (1% yeast extract powder, 2% peptone, 2% glucose, 2% agar) from th...
Embodiment 3
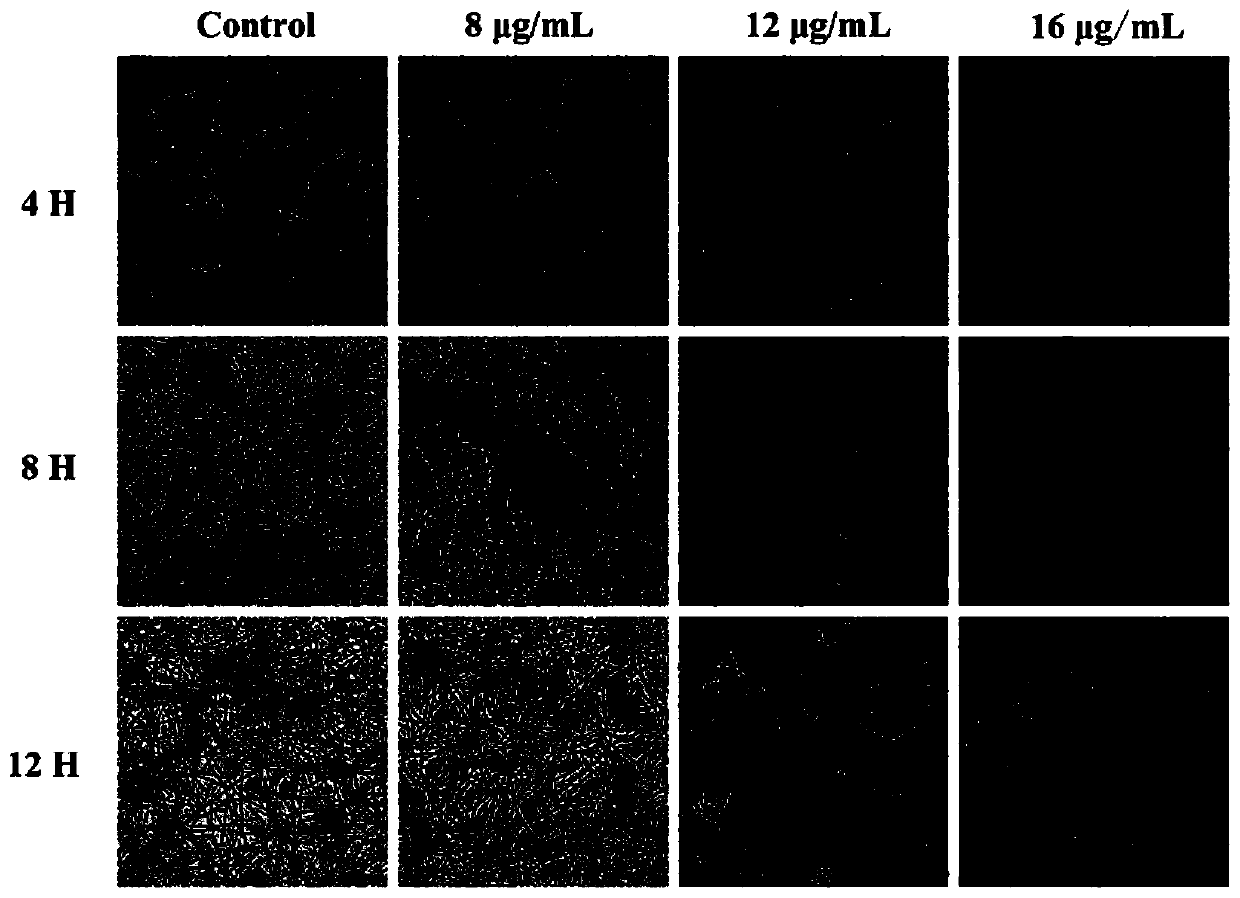
[0046] Embodiment 3Effect of romania saponin A on the virulence of Candida albicans
[0047] 1.Effect of roechoside A on the adhesion ability of Candida albicans
[0048] Dilute the YEM30 strain in the logarithmic growth phase to about 1×10 with RPMI1640 medium 6 cells / mL, add Erythroside A to the final concentrations of 2, 4, 8, and 16 μg / mL, respectively, and amphotericin B with a final concentration of 2 μg / mL was used as the positive control group, and the solvent DMSO with a final concentration of 1% was used as the negative control group , transfer 100 μL / well of each treatment group into a flat-bottomed 96-well plate, set up 4 parallel wells for each treatment group, incubate at 37°C for 90 minutes, discard the supernatant, wash the bottom of the plate three times with sterile PBS buffer, remove For non-adherent cells, the amount of cells adhered to the bottom of the well plate was detected with the XTT cell proliferation assay kit. The experimental results are shown ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com