Glucan-based nanogel and preparation method and application thereof
A nano-gel, dextran-based technology is applied in the field of dextran-based nanogel and its preparation to achieve the effects of reducing toxic side effects, improving anti-tumor effect, and reducing tumor recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
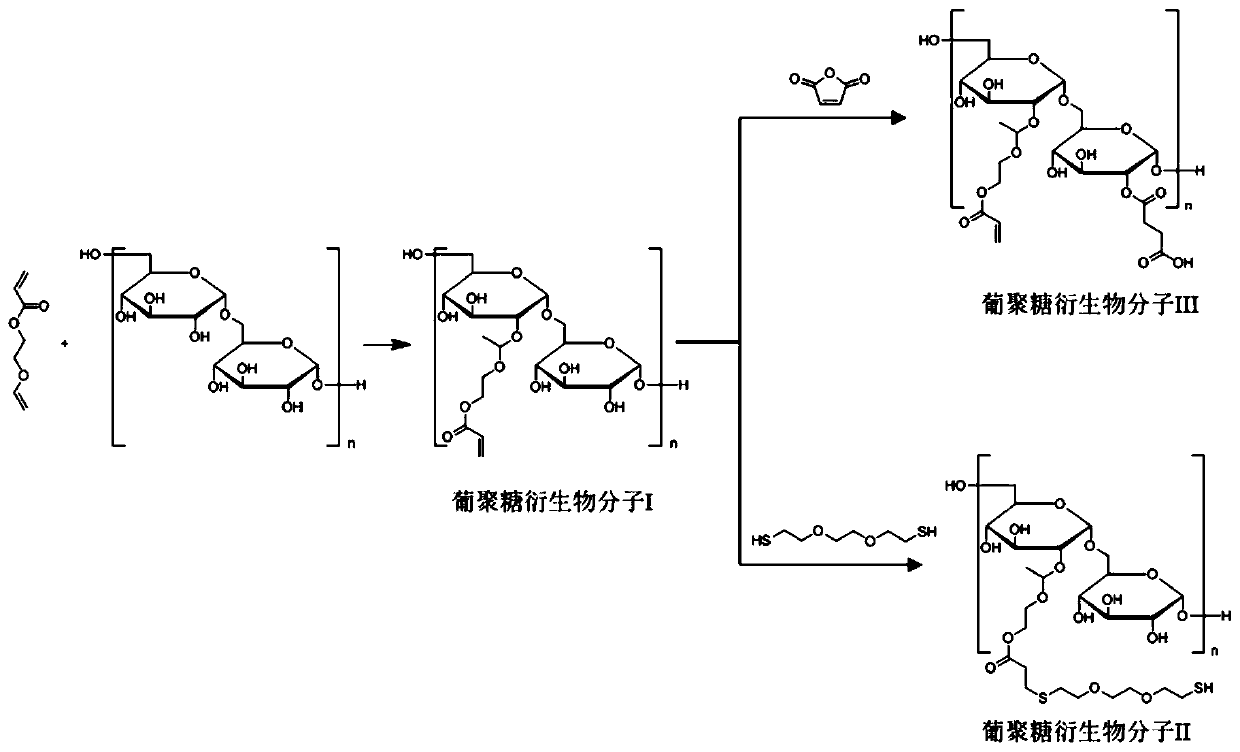
[0047] (1) Synthesis of Dextran Derivative I Modified by Vinyl Ether Acrylate
[0048] Dimethyl sulfoxide was distilled to remove water in advance for later use. Dextran with a molecular weight of 20,000 was dissolved in ultrapure water and freeze-dried for later use. Weigh 1.25g of lyophilized dextran, add 65mL of anhydrous dimethyl sulfoxide and properly heat up to dissolve it, vacuumize for 10 minutes to remove water and oxygen, and add 125mg of p-toluenesulfonic acid ( PSTA) and 1 mL of vinyl ether acrylate. After the whole system was magnetically stirred and reacted for 6 hours under the condition of anhydrous and oxygen-free normal temperature, 2-3 drops of triethylamine were added to quench the reaction. The reaction solution was transferred to methanol for dialysis overnight, methanol was removed by rotary evaporation, the remaining reaction solution was added dropwise to a sufficient amount of glacial ether to precipitate, and the precipitated white solid particles ...
Embodiment 2
[0057] Example 2 Acid degradation behavior verification of nanogel
[0058] Take 2mL of 1.0mg / mL dextran-based nanogel prepared in the same batch, divide it into two parts, place 1mL each in a dialysis bag with a molecular weight cut-off of 3500, and place them in 20mL buffer media with different pH, 37±0.5 Under shaking at ℃, samples were taken at 0h, 1h, 3h, 7h, 24h, 48h, and 72h to detect the particle size of the sample, and the results were as follows image 3 As shown in (a), it was found that the particle size of the dextran-based nanogel remained unchanged in the buffer solution of pH 7.4, and it was still uniform and stable for 72 hours, while in the buffer solution of pH 5.0, the system was chaotic and disintegrated after 7 hours Degradation flocculation occurred, which evidenced that the acid-responsive acetal groups were hydrolyzed in weak acid, degrading the gel network.
Embodiment 3
[0059] Example 3 Doxorubicin In Vitro Acid Response Release Experiment
[0060] Taking doxorubicin-loaded dextran-based nanogel (dosing amount 0.05:1) as an example, the acid-responsive release behavior of the dextran-based nanogel in vitro was studied. Take 2mL of dextran-based nanogels loaded with doxorubicin at 1mg / mL prepared in the same batch (the entrapment efficiency is 35.56% as measured by the ultraviolet standard curve method), divide into two, and each 1mL solution is placed in the molecular weight cut-off In the dialysis bag of 3500, respectively put in the dissolution container filled with 20mL dissolution medium, avoid light, and carry out the in vitro release test of doxorubicin at the medium temperature of 37±0.5°C. According to the set time interval 0h, 0.5h, 1h, 2h, 3h, 4h, 6h, 17h, 24h, take out 2mL of the release solution outside the dialysis bag, and add the same volume of blank dissolution medium, and measure the doxorubicin in the release solution by flu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com