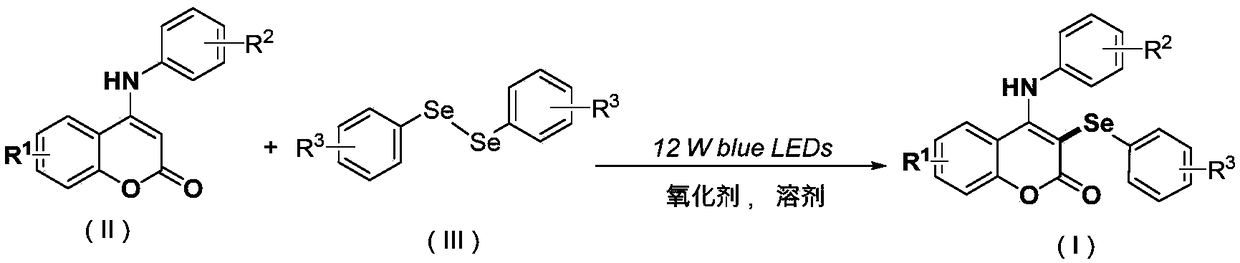
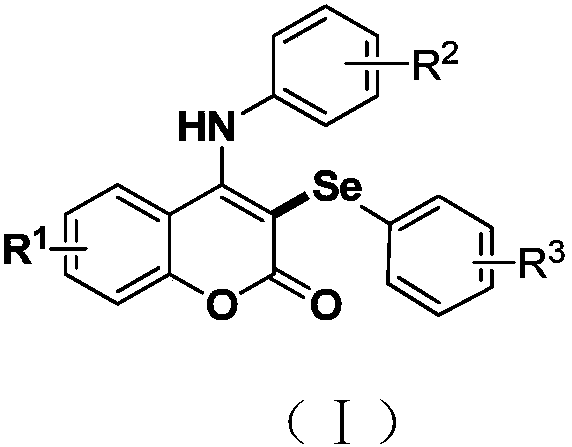
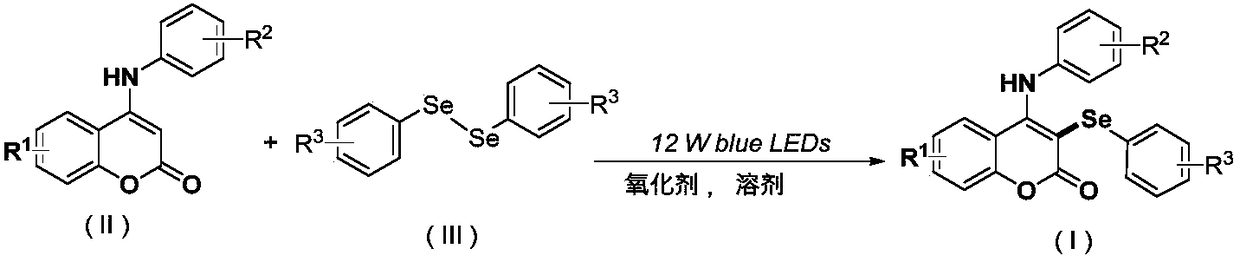
Synthetic method of visible accelerant C-3-site aryl-seleno substituent coumarin
A synthetic method, visible light technology, applied in the direction of organic chemistry, etc., to achieve the effects of low environmental pollution, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] At room temperature, ammonium peroxodisulfate (0.4 mmol), C-4 aniline substituted coumarin (0.2 mmol), diphenyl Diselenide (0.15 mmol), acetonitrile (2 ml), and a 12-watt blue LED lamp were placed 3 cm away from the reaction tube, and reacted at room temperature for 24 h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specification: 200-300 mesh, eluent: petroleum ether / ethyl acetate (10:1, v / v)) to obtain 63 mg of the target product with a yield of 75%.
[0048] The obtained product nuclear magnetic spectrum data are: 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.88(s,br,1H),7.47-7.44(m,3H),7.35(d,1H,J=8.3Hz),7.31(dd,2H,J=7.6Hz,8.0Hz ),7.24-7.18(m,5H),7.00(d,2H,J=8.0Hz),6.93(t,1H,J=7.3Hz).
[0049] 13 C NMR (CDCl...
Embodiment 2
[0052]
[0053] At room temperature, ammonium peroxodisulfate (0.4 mmol), C-4 aniline substituted coumarin (0.2 mmol), diaryl Diselenide (0.15 mmol), acetonitrile (2 ml), and a 12-watt blue LED lamp were placed 3 cm away from the reaction tube, and reacted at room temperature for 24 h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specification: 200-300 mesh, eluent: petroleum ether / ethyl acetate (15:1, v / v)) to obtain 61 mg of the target product with a yield of 76%.
[0054] The obtained product nuclear magnetic spectrum data are: 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.85(s,br,1H),7.45(t,1H,J=8.2Hz),7.35-7.24(m,5H),7.21-7.18(m,2H),7.10(t, 1H, J=7.6Hz), 7.00-6.98(m, 3H), 6.93(t, 1H, J=8.3Hz), 2.24(s, 3H).
[0055] 13 C NM...
Embodiment 3
[0058]
[0059] At room temperature, add ammonium peroxodisulfate (0.2 mmol), sodium peroxodisulfate (0.3 mmol), C-4 aniline substituted coumarin into a 25 ml Schlenk tube equipped with a magnetic stirrer Chlorine (0.2 mmol), diaryldiselenide (0.2 mmol), 1,2-dichloroethane (2 ml), a 12-watt blue LED lamp placed 3 cm away from the reaction tube, at room temperature Reaction 24h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200-300 mesh, eluent: petroleum ether / ethyl acetate (15:1, v / v)) to obtain 65 mg of the target product with a yield of 76%.
[0060] The obtained product nuclear magnetic spectrum data are: 1 H NMR (CDCl 3 ,500MHz,ppm)δ7.85(s,br,1H),7.48(t,1H,J=7.0Hz),7.39-7.31(m,5H),7.23(d,1H,J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com