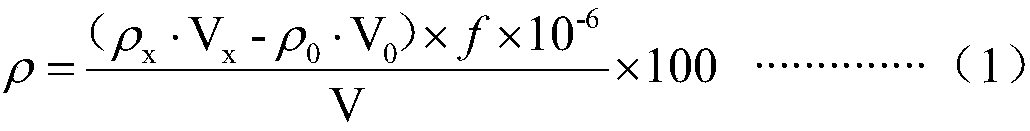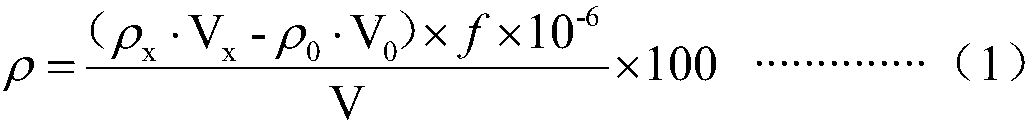Measuring method of copper, plumbum, zinc, manganese, cadmium and chromium in cyaniding tail liquid
A technique for the determination of lead, zinc, manganese, cadmium, chromium, and a method for determining chromium, copper, zinc, lead, manganese and cadmium in the tail liquid of cyanidation process in the gold industry, can solve the problem that metal ions cannot be quickly and accurately determined, etc. Achieving the effect of avoiding human injury, accurate method and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The unknown cyanide tail liquid of a gold enterprise was selected as the experimental object, and the metal ions copper, lead, zinc, manganese, cadmium and chromium were determined on it.
[0036](1) Accurately measure 25mL of cyanide tail liquid V with a measuring cylinder, place it in a 300mL conical flask, add 0.5g of potassium chlorate, 0.2g of ammonium bifluoride, connect the hydrogen cyanide absorption device, add 5mL of nitric acid at the acid inlet, and immediately Cover the acid inlet, shake well, place on the electric heating plate and heat for 5 minutes, the hydrogen cyanide produced is fully absorbed by the sodium hydroxide absorption solution at the right end, then add 10mL nitric acid through the acid inlet, cover and place on the electric heating plate Heat and digest until the remaining volume is 8mL, take it off and cool slightly, and remove the hydrogen cyanide absorption device;
[0037] The above operations were all carried out under a fume hood, and...
Embodiment 2
[0045] The unknown cyanide tail liquid of another gold enterprise was selected as the experimental object, and the metal ions copper, lead, zinc, manganese, cadmium and chromium were determined on it.
[0046] (1) Accurately measure 25mL of cyanide tail liquid V with a measuring cylinder, place it in a 300mL conical flask, add 0.8g of potassium chlorate and 0.3g of ammonium bifluoride, connect the hydrogen cyanide absorption device, add 7mL of nitric acid at the acid inlet, and immediately Cover the acid inlet, shake well, place on the electric heating plate and heat for 7 minutes, the hydrogen cyanide produced is fully absorbed by the sodium hydroxide absorption solution at the right end, then add 13mL nitric acid through the acid inlet, cover and place on the electric heating plate Heat and digest until the remaining volume is about 10mL, then remove it to cool slightly, and remove the hydrogen cyanide absorption device;
[0047] The above operations were all carried out und...
Embodiment 3
[0055] Include the following steps:
[0056] (1) Accurately measure 25mL of cyanide tail liquid V with a graduated cylinder, place it in a 300mL conical flask, add 1g of potassium chlorate, 0.5g of ammonium bifluoride, connect the hydrogen cyanide absorption device, add 8mL of nitric acid at the acid inlet, and immediately Cover the acid inlet, shake well, place on the electric heating plate and heat for 10 minutes, the hydrogen cyanide produced is fully absorbed by the sodium hydroxide absorption solution at the right end, then add 15mL nitric acid through the acid inlet, cover and place on the electric heating plate Heat it, and when it is digested to a volume of 10mL, remove it to cool slightly, and remove the hydrogen cyanide absorption device;
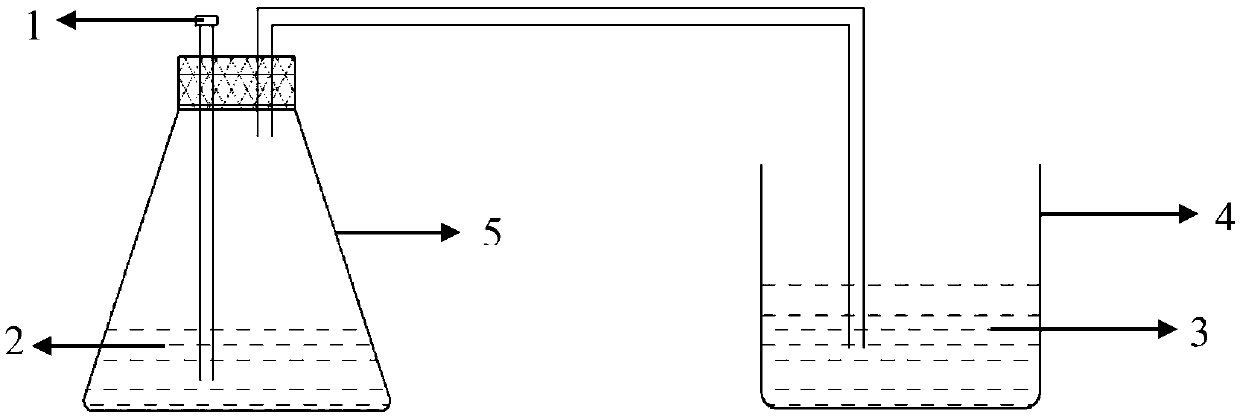
[0057] The above operations were all carried out under a fume hood, and personal protective equipment was worn to avoid personal injury caused by hydrogen cyanide. figure 1 As shown, acid addition port 1, reaction solution 2, sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com