Acid-sensitive doxorubicin prodrug based on zwitterion and folic acid targeting and its preparation method and application
A zwitterion and folic acid targeting technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of toxic side effects of healthy tissues, poor drug targeting, and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
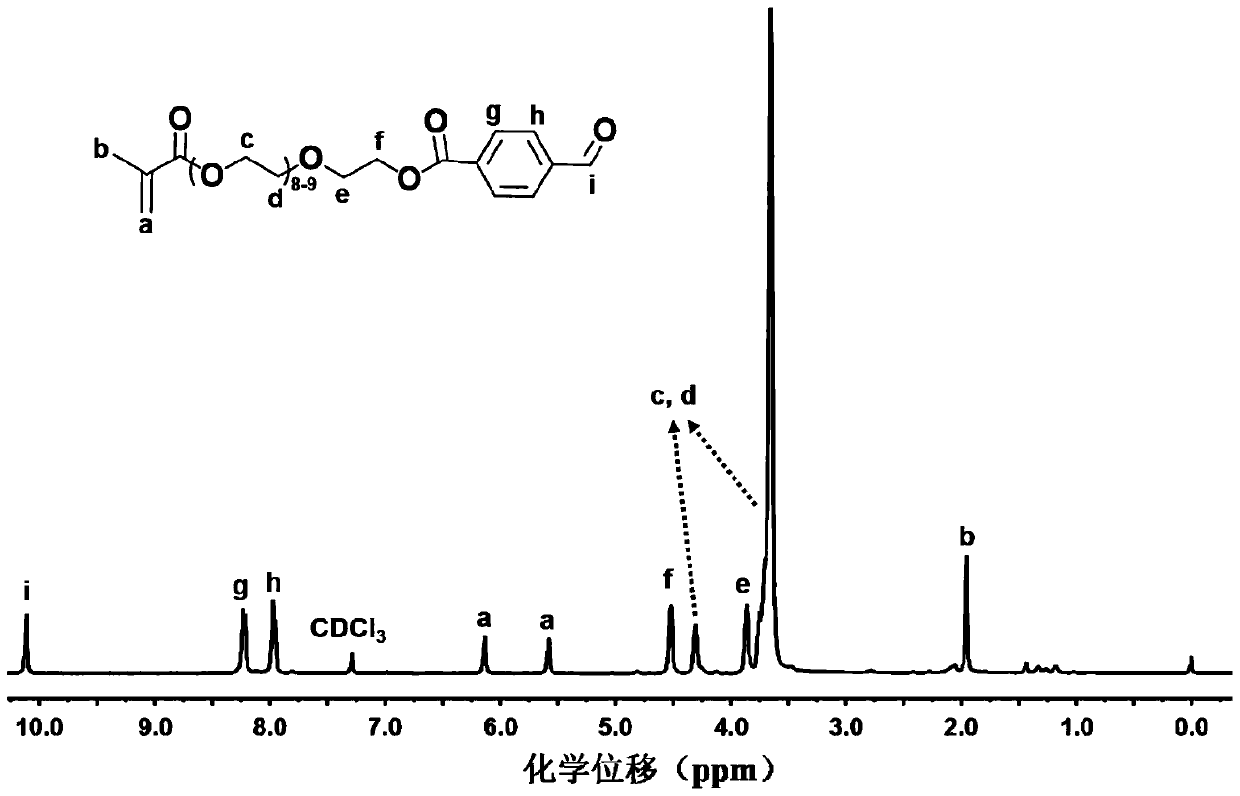
[0082] Embodiment one: the synthesis of methacrylate polyethylene glycol p-aldehyde benzoate (PEGMA-BZ)
[0083] First, under the condition of inert gas atmosphere, methacrylate polyethylene glycol (PEGMA-OH) and p-aldehyde benzoic acid were used as raw materials to N, N' -Diisopropylcarbodiimide is used as water absorbing agent and 4-dimethylaminopyridine is used as catalyst, and methacrylate polyethylene glycol p-aldehyde benzoate (PEGMA-BZ) is obtained through esterification reaction. The specific synthesis method is as follows: put a 250mL branched round bottom flask with a stirrer in an oven at 120°C for 24 h, take it out, plug it with a glass stopper, connect it to an oil pump through a latex tube, and evacuate the branched round bottom flask to room temperature. , and then into high-purity nitrogen. During aeration, polyethylene glycol methacrylate (10.0 g, 0.02 mol) and p-aldehyde benzoic acid (6.0 g, 0.04 mol) were added, and 100 mL of dried tetrahydrofuran (THF ) ...
Embodiment 2
[0085] Embodiment two: poly (2-methacryloyloxyethyl phosphorylcholine- co -Synthesis of methacrylate polyethylene glycol p-aldehyde benzoate) copolymer
[0086] Dry the 50 mL round-bottomed flask and glass stopper with a stirring bar in an oven at 120 °C for 24 h, take it out, plug it with a glass stopper, and connect it to an oil pump through a latex tube. into high-purity nitrogen. During aeration, (4-cyanopentanoic acid) trithioacetate (CEP) (10 mg, 0.038 mmol), methacrylate polyethylene glycol p-aldehyde benzoate (1.90 g, 3.04 mmol) and 2-methacryloyloxyethylphosphorylcholine (1.12 g, 3.80 mmol); add 16 mL of dimethyl sulfoxide and deionized water to the branched flask (V / V=1: 1) Mix the solution, then pass high-purity nitrogen gas, vacuumize, repeat this three times and then fill with nitrogen gas. Stir until completely dissolved, then transfer to a 70°C oil bath to react for 12 h.
[0087] The reaction was terminated by rapid cooling. A dialysis bag with a molecular...
Embodiment 3
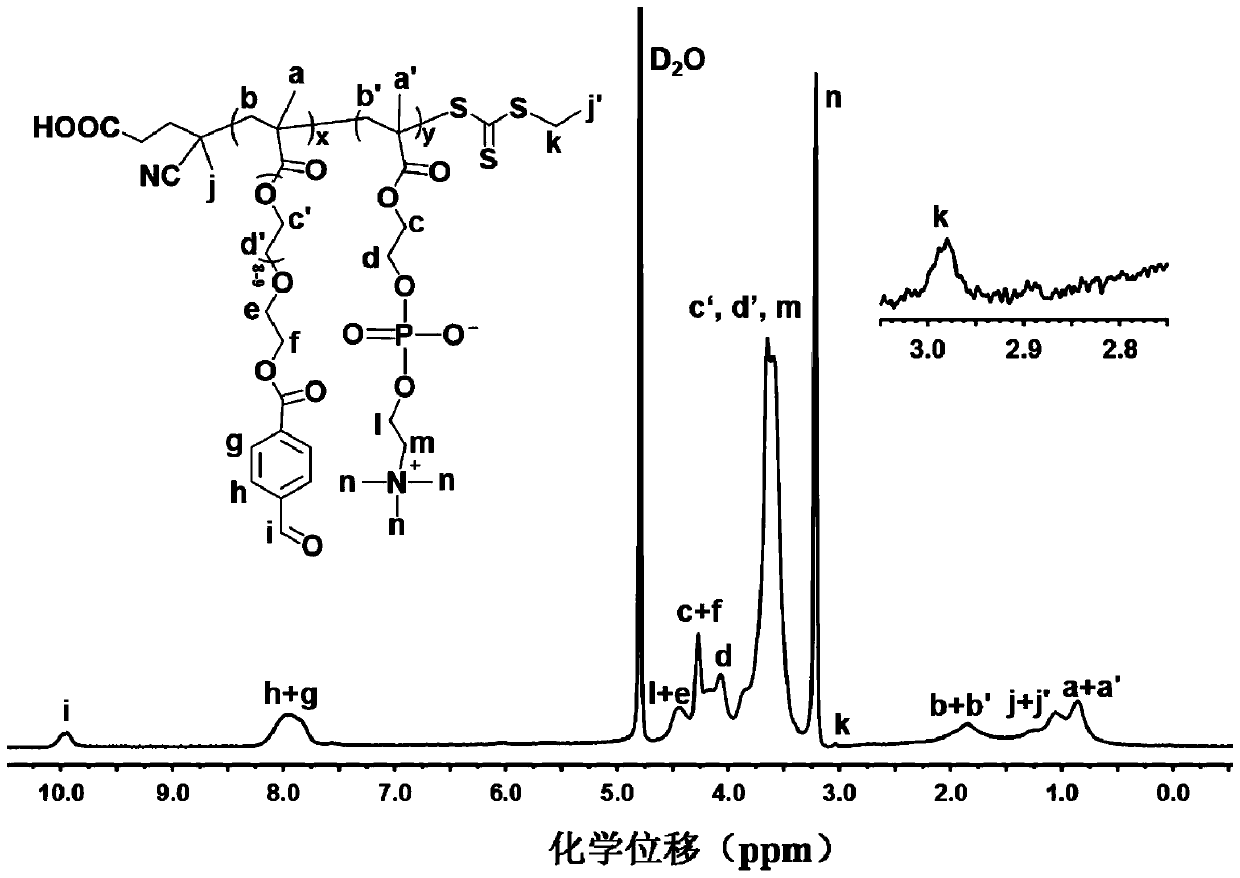
[0088] Embodiment three: poly (2-methacryloyloxyethyl phosphorylcholine- co -Synthesis of methacrylate polyethylene glycol p-aldehyde benzoate) prodrug
[0089] Add P(MPC- co -PEGMA-BZ) (150 mg, 0.0025 mmol), doxorubicin hydrochloride (80 mg, 0.147 mmol) and 0.5 mL of triethylamine, then add 10 mL of the same amount of dimethyl sulfoxide and deionized water ( V / V=1: 1), after ultrasonication for 10 min, transfer to 30°C oil bath for 48 h. After the reaction is over, dialyze with ultrapure water for 72 hours, and use ammonia water to adjust the pH of the dialyzed aqueous solution to alkaline. fracture. Finally, the solution in the dialysis bag was freeze-dried to obtain a dark red polymer-doxorubicin prodrug called P(MPC- co -PEGMA-BZ)- g -DOX. The productive rate is 78.3%, and the proton nuclear magnetic resonance spectrum figure of product is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com