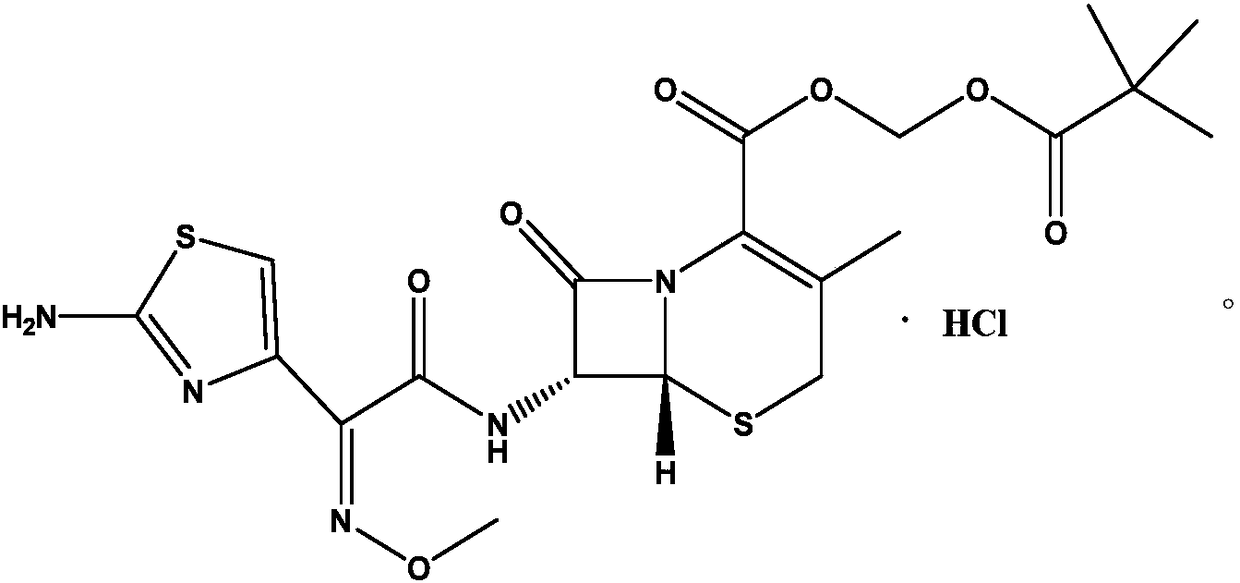
Pharmaceutical composition containing cefetamet pivoxil hydrochloride
A technology of ceftamet pivoxil hydrochloride and composition, which is applied in the field of pharmaceutical composition containing ceftamet pivoxil hydrochloride and its preparation, and can solve problems such as gelation, poor drug dissolution, and high requirements for fillers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Embodiment 1 Ceftazidime pivoxil hydrochloride film-coated tablet preparation (unit: g)
[0125] prescription:
[0126]
[0127] The preparation process is as follows:
[0128] 1) Get ceftazime pivoxil hydrochloride crude drug and pulverize, cross 200 mesh sieves, set aside;
[0129] 2) Take the filler mannitol, 50% of the prescription amount of the disintegrating agent low-substituted hydroxypropyl cellulose, sieve, and set aside;
[0130] 3) Take the prescribed amount of ceftazidime hydrochloride, filler mannitol and 50% of the prescribed amount of disintegrating agent low-substituted hyprolose, mix well, dry granulate, and granulate to obtain drug-loaded ceftazidime hydrochloride granules ;
[0131] 4) Take the drug-loaded ceftazidime pivoxil hydrochloride granules, the remaining 50% of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose, the gel inhibitor sodium cyclamate, and the lubricant magnesium stearate, and mix them evenl...
Embodiment 2
[0134] Embodiment 2 Ceftamet pivoxil hydrochloride hard capsule preparation (unit: g)
[0135] prescription:
[0136]
[0137] The preparation process is as follows:
[0138] 1) Get ceftazime pivoxil hydrochloride crude drug and pulverize, cross 200 mesh sieves, set aside;
[0139] 2) Take the filler mannitol, 50% of the prescription amount of the disintegrating agent low-substituted hydroxypropyl cellulose, sieve, and set aside;
[0140] 3) Take the prescribed amount of ceftazidime hydrochloride, filler mannitol and 50% of the prescribed amount of disintegrating agent low-substituted hyprolose, mix well, dry granulate, and granulate to obtain drug-loaded ceftazidime hydrochloride granules ;
[0141] 4) Take the drug-loaded ceftazidime pivoxil hydrochloride granules, the remaining 50% of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose, the gel inhibitor sodium cyclamate, and the lubricant magnesium stearate, and mix them evenly to obta...
Embodiment 3
[0143] Embodiment 3 preparation of ceftazidime pivoxil hydrochloride granules (unit: g)
[0144] prescription:
[0145]
[0146] The preparation process is as follows:
[0147] 1) Get ceftazime pivoxil hydrochloride crude drug and pulverize, cross 200 mesh sieves, set aside;
[0148] 2) Take the filler mannitol, 50% of the prescription amount of the disintegrating agent low-substituted hydroxypropyl cellulose, sieve, and set aside;
[0149] 3) Take the prescribed amount of ceftazidime hydrochloride, filler mannitol and 50% of the prescribed amount of disintegrating agent low-substituted hyprolose, mix well, dry granulate, and granulate to obtain drug-loaded ceftazidime hydrochloride granules ;
[0150] 4) Take the drug-loaded ceftazidime pivoxil hydrochloride granules, the remaining 50% of the prescription amount of the disintegrant low-substituted hydroxypropyl cellulose, the gel inhibitor sodium cyclamate, and the lubricant magnesium stearate, and mix them evenly to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com