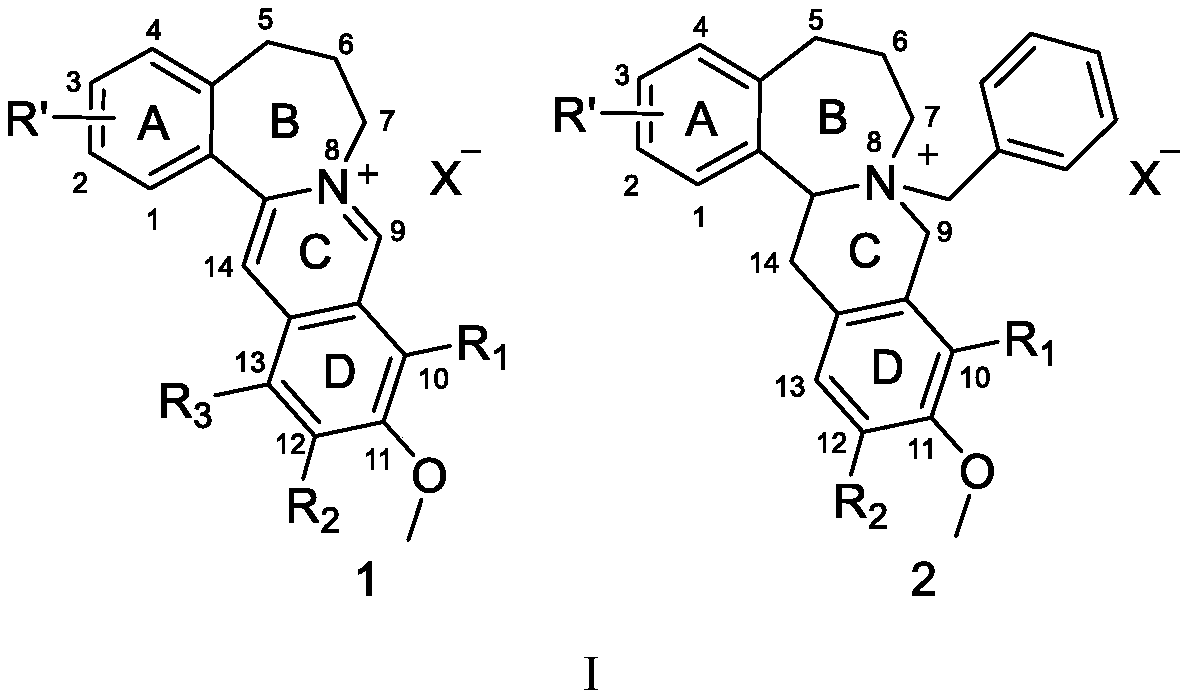
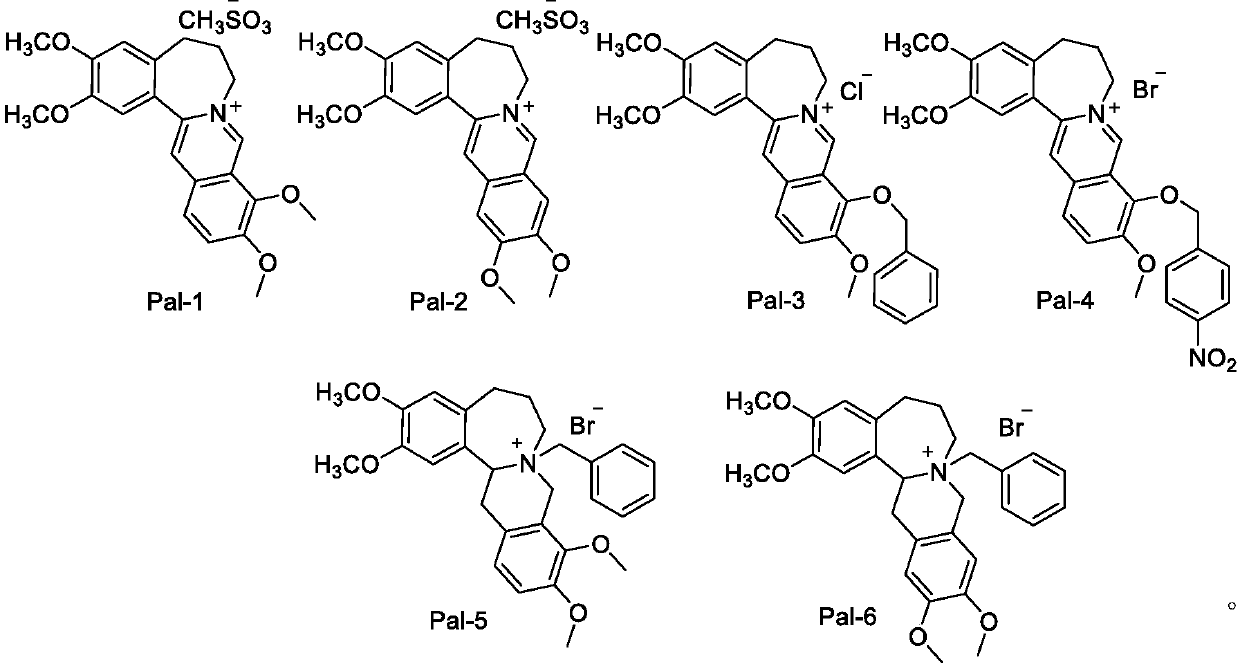
Synthesis of high-b-cyclic berberine and palmatine derivatives and their use as hypoglycemic agents
A derivative, berberine technology, applied in the field of compound drugs, can solve the problems of poor solubility of berberine, unsatisfactory, low oral bioavailability, etc., and achieve good medicinal potential, mild reaction conditions, and efficacy. clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the synthesis of methanesulfonic acid high B ring berberine (Ber-1)
[0038]
[0039] As shown in the above synthetic route: with 3,4-(methylenedioxy)benzaldehyde as the initial raw material, the carbon chain of the B ring is extended by construction, and then the C ring D ring isoquinoline mother ring is constructed, and finally the B ring is cyclized. ring to obtain the target compound methanesulfonic acid homo-B-ring berberine (Ber-1).
[0040] Synthesis of compound 2b:
[0041] In a 250ml round bottom flask, dissolve compound 1b (12g, 79.9mmol), malonic acid (16.6g, 159.9mmol) with 7.2ml piperidine and 140ml pyridine, then move the reaction solution to an oil bath at 115°C and heat to reflux for about 2 hours. The reaction process was monitored by TLC until the reaction of compound 1b was complete. After the reaction solution was cooled to room temperature, the reaction solution was slowly poured into 200 ml of hydrochloric acid solution (2 mol / L...
Embodiment 2
[0064] Embodiment 2, the synthesis of high B ring berberine hydrochloride (Ber-2)
[0065]
[0066] As shown in the above synthetic route: the high B-ring berberine hydrochloride (Ber-2) was obtained by alkalinizing and hydrochlorizing methanesulfonic acid high-B-ring berberine (Ber-1) as a starting material.
[0067] Synthesis of compound 12b:
[0068] Dissolve Ber-1 with 6 ml of methanol / water mixed solvent (V / V=5 / 1) at room temperature, and then slowly add calcium oxide solid therein to adjust the pH of the reaction system to 9-10. Move the reaction solution to a 60°C oil bath and heat it for 3 hours, then pour the reaction solution into 10ml of water, extract the water layer 4 times with dichloromethane, combine the dichloromethane layers, wash with saturated saline, and wash with anhydrous sodium sulfate Dry, filter and concentrate under reduced pressure to obtain the crude product 12b, which is directly reacted in the next step without further purification.
[0069]...
Embodiment 3
[0072] The synthesis of embodiment 3, Ber-3
[0073]
[0074]As shown in the above synthetic route: use compound 7b as the starting material to couple with 2-bromo-4,5-dimethoxybenzaldehyde (compound 8') through the Sonogashira reaction, and then through the ring closure of the isoquinoline ring and Ring closure of the high B ring yields compound Ber-3.
[0075] Synthesis of compound 9b':
[0076] Similar to the synthesis of compound 9b in Example 1.
[0077] Put compound 7b, 2-bromo-4,5-dimethoxybenzaldehyde 8', bistriphenylphosphine palladium dichloride and cuprous iodide in a 50ml double-necked bottle, and replace the gas with argon three times. Dissolve it with triethylamine and place it at 70°C for three hours. The reaction process was monitored by TLC until the reaction of compound 7b was complete. The reaction solvent was spun off under reduced pressure, and the residue was redissolved in ethyl acetate and washed with water. The aqueous layer was extracted twice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com