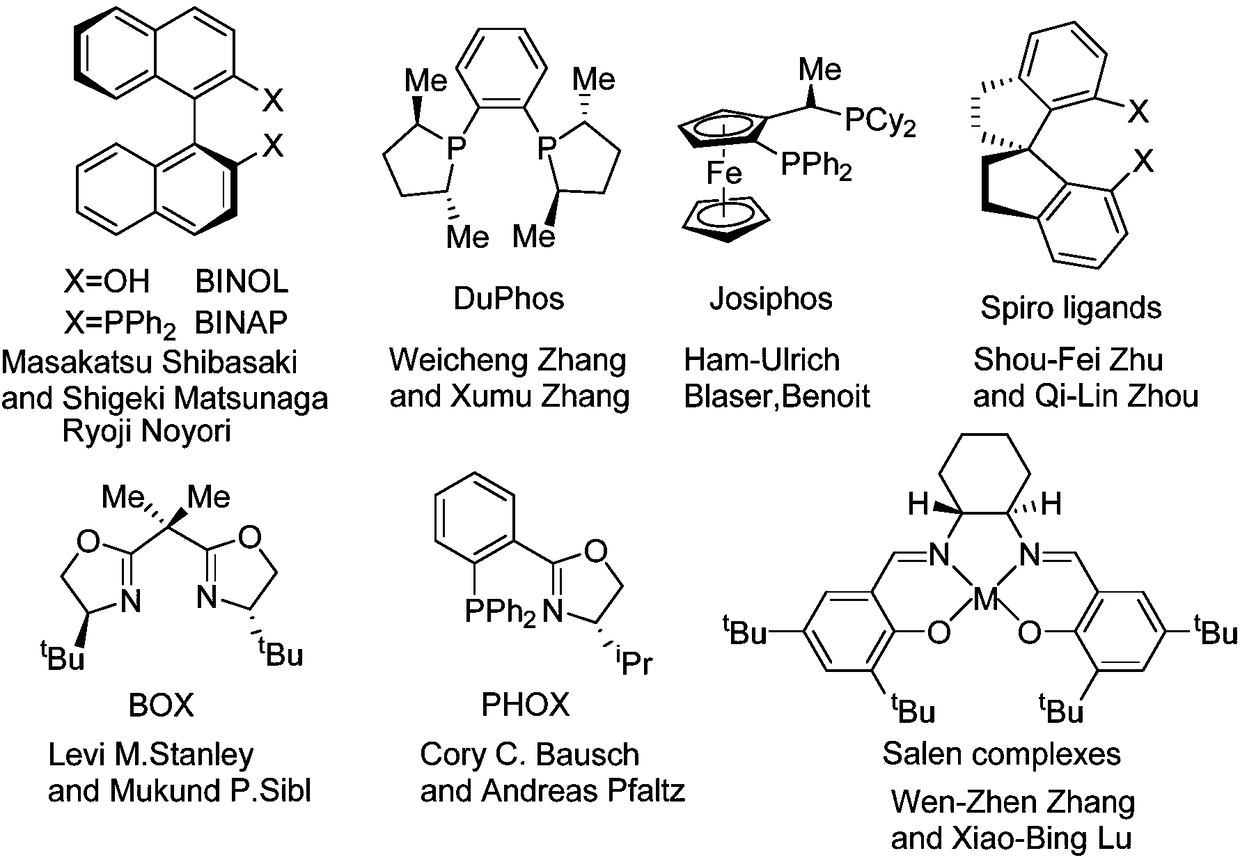
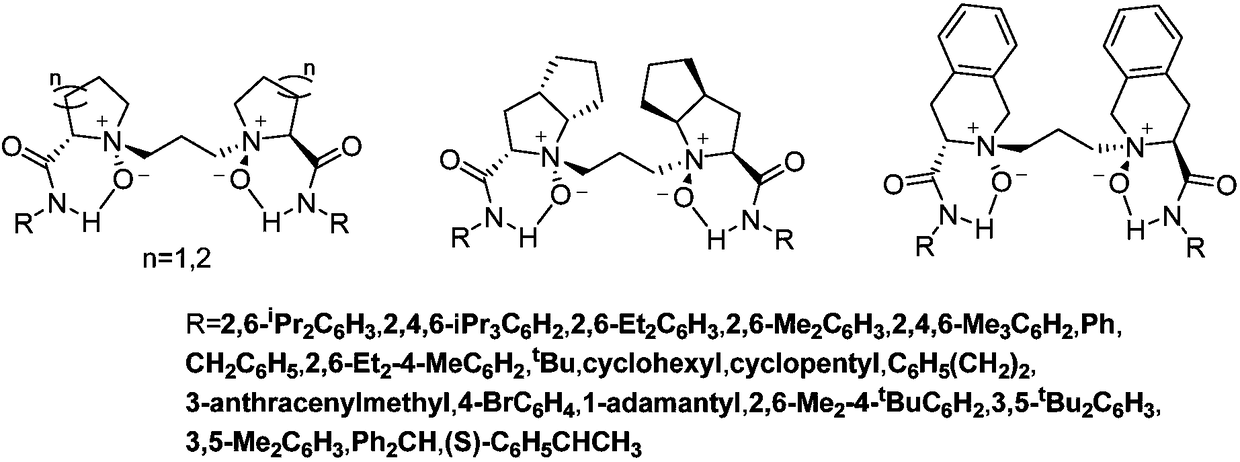
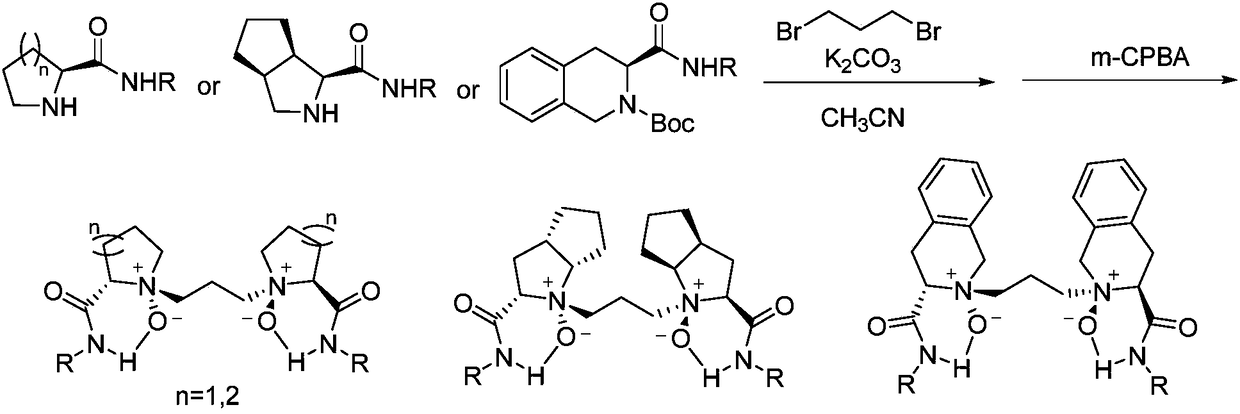
Novel chiral nitrogen oxygen ligands and synthesis method thereof
A chiral, nitrogen-oxygen technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1: Synthesis of Compound L from Compound 2a 4 -RaR
[0127] Add 2,5-dimethoxytetrahydrofuran (1eq) into the reaction vessel, add a small amount of dilute hydrochloric acid, raise the temperature, and hydrolyze it into succinic dialdehyde. Dissolve the succinic dialdehyde obtained above in 1,2-dichloroethane, then add compound 2a (2eq), mix and stir for 15min, continue to add sodium triacetoxyborohydride (2.4eq), stir at room temperature for 12h, slowly add Saturated sodium bicarbonate until no bubbles are generated, extracted and separated with dichloromethane, purified by vacuum distillation column chromatography, and obtained a white solid product. After that, 2b was dissolved in dichloromethane, cooled to -20°C, and m-chloroperoxy Benzoic acid (2.2eq), after stirring for 30min, warm up to room temperature, add saturated sodium bicarbonate, stir for 5 minutes, add dichloromethane for extraction and separation, vacuum distillation column chromatography (ethyl ...
Embodiment 2
[0128] Example 2: Synthesis of Compound L from Compound 2a 6 -RaR
[0129] Add 1,2-cyclohexanediol (1eq) into the reaction vessel, add solvent 1,2-dichloroethane, cool at 0°C, slowly add sodium periodate (2eq) and sodium bicarbonate (2.1eq), Stir at room temperature for 24h, filter, wash with a small amount of solvent, add the obtained filtrate to the stirrer, then add compound 2a (2eq), after stirring for 15min, continue to add sodium triacetoxyborohydride (2.4eq), stir at room temperature for 12h, slowly add Saturated with sodium bicarbonate until no bubbles were generated, extracted and separated with dichloromethane, and purified by column chromatography under reduced pressure to obtain the white solid product 2c. Follow-up operation is referring to embodiment 1.
Embodiment 3
[0130] Example 3: Synthesis of Compound L from Compound 2a 7 -RaR
[0131] Add compound 2a (2eq) to the reaction vessel, add the solvent acetonitrile to dissolve, add 1,7-dibromoheptane (1eq), add diisopropylethylamine (4eq) and sodium iodide (0.2eq), heat Reflux at 100° C. for 12 h, cool to room temperature and suction filter, and conduct vacuum distillation column chromatography of the obtained filtrate (ethyl acetate:petroleum ether=1:1) to obtain the white solid product 2d. Follow-up operation is referring to embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com