Photo-induced dimer type chimeric antigen receptor
A chimeric antigen receptor, light-induced technology, applied in the field of biomedical transformation, can solve problems such as the publication of research results, and achieve the effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1, Design of Light-Induced Dimer Type Recombination Chimeric Antigen Receptor
[0052] In the present invention, the CD19-CAR molecule, which is currently the most widely studied and used molecule, is selected as an example to construct the first monomer and the second monomer of light-induced dimer recombination and antigen receptor respectively. A total of two groups of light-induced dimer-type chimeric antigen receptors were designed, named combination 1 and combination 2 respectively (for the sequence structure, see figure 1 ).
[0053] Combination 1 first monomer components are as follows: CD8α signal peptide (amino acid sequence shown in SEQ ID No: 2, nucleotide sequence shown in SEQ ID No: 1), anti-CD19 scFv (amino acid sequence shown in SEQ ID No: 4, the nucleotide sequence is shown in SEQ ID No: 3), CD8 hinge (the amino acid sequence is shown in SEQ ID No: 6, the nucleotide sequence is shown in SEQ ID No: 5), CD8 transmembrane (amino acid The sequence...
Embodiment 2
[0065] Example 2, Light Induced Expression of Dimeric Chimeric Antigen Receptor
[0066] 1. Construction of co-expression vector
[0067] Lenti-EF1α plasmid vector was purchased from iCartab Biomedical Technology (Suzhou) Co., Ltd. (http: / / www.icartab.com.cn).
[0068] According to the sequences encoded by SEQ ID NO: 35 and SEQ ID NO: 40, Beijing Aoke Biotechnology Co., Ltd. was entrusted to carry out the whole gene synthesis, and then inserted into the EcoRI and BamHI restriction sites of the Lenti-EF1α plasmid. Transformed into E.coli (TOP10, purchased from Quanshijin Company), after correct sequencing, the plasmid was extracted and purified using Qiagen’s plasmid purification kit, and the endotoxin-free plasmid of the two combined recombinant expression vectors was obtained, that is, Lenti- LID-CD19CAR-1 and Lenti-LID-CD19CAR-2.
[0069] 2. Packaging of lentivirus vector Lentivirus
[0070] Inoculate 5 × 10 in gelatin-precoated 15-cm dishes 6 HEK 293T cells were cultur...
Embodiment 3
[0076] Example 3, Analysis of cell killing activity of LID CD19-CAR Jurkat T cells
[0077] (1) Detection of IL-2 secretion
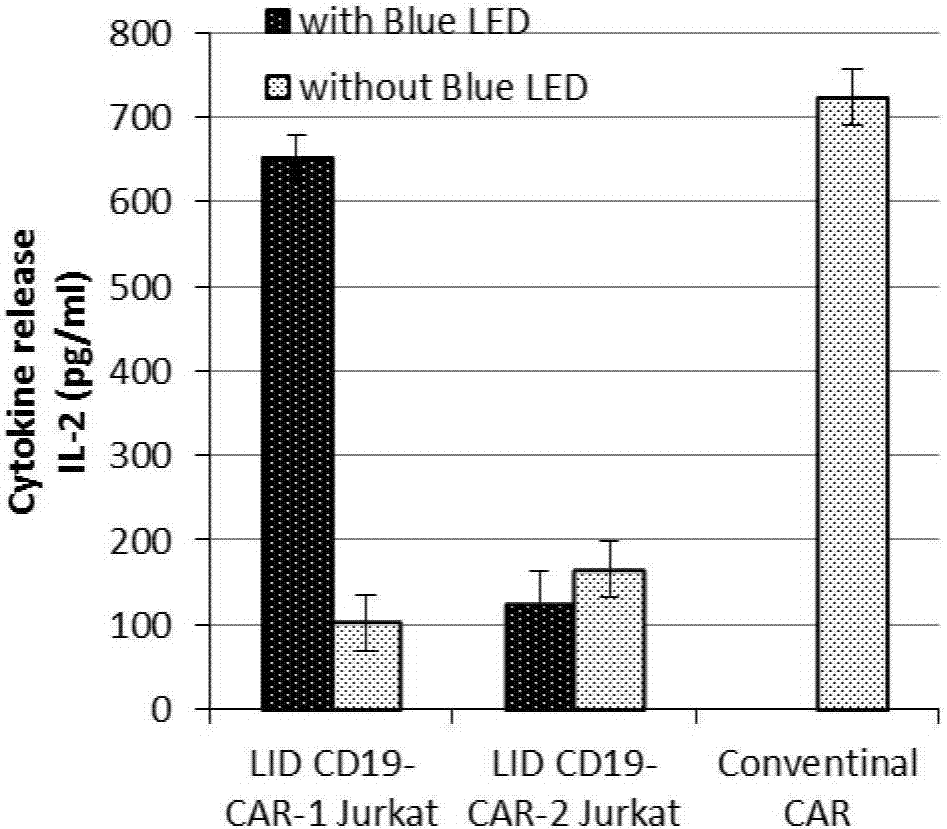
[0078] The degree of activation of immune cells was evaluated by the level of IL-2 secreted by cells. Combine K562 and K562-CD19 (K562 cells obtained by transfection with stable CD19 phenotype) at 1×10 5 The concentration per well was plated in a 96-well plate, and 100 μl of target cells were added to each well. The LID CD19-CAR Jurkat T cells (LID CD19-CAR-1 Jurkat T cells and LIDCD19-CAR-2 Jurkat T cells) according to the ratio of target cells: effector cells = 1:1, 1:3, 1:10, respectively co-culture with target cells. Set both illuminated and non-illuminated groups. When co-cultivating in the light group, blue LED light (1.2mW / cm 2 at450nm) was irradiated for 6h; the non-illumination group wrapped the cell culture plate with tinfoil. At 37°C, 5% CO 2 After 18-20 hours of co-cultivation in the incubator, the cell culture suspensions were colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com