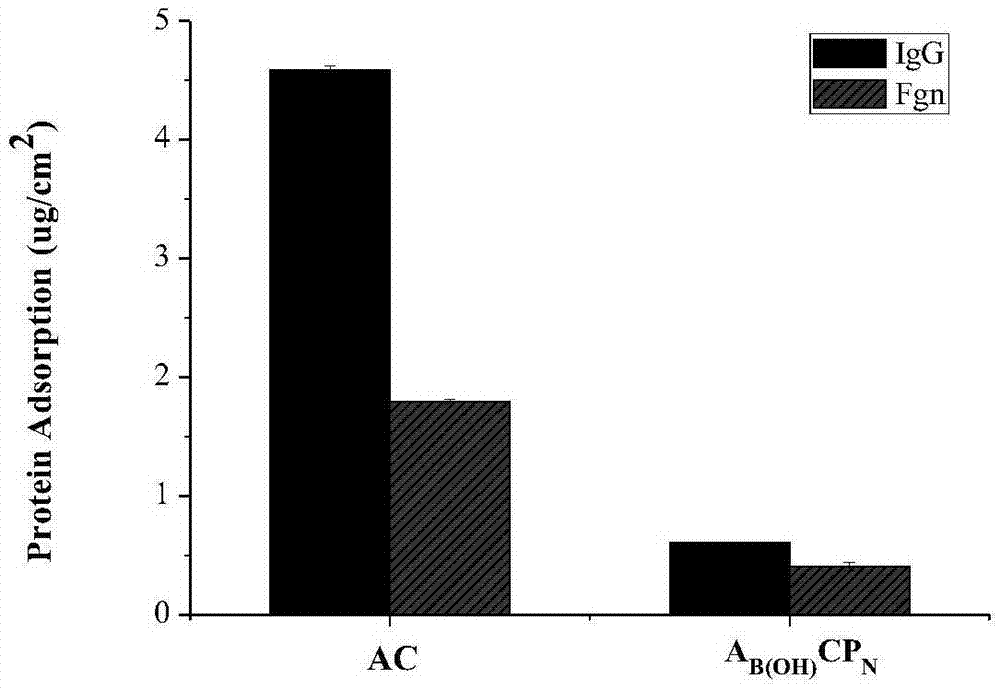
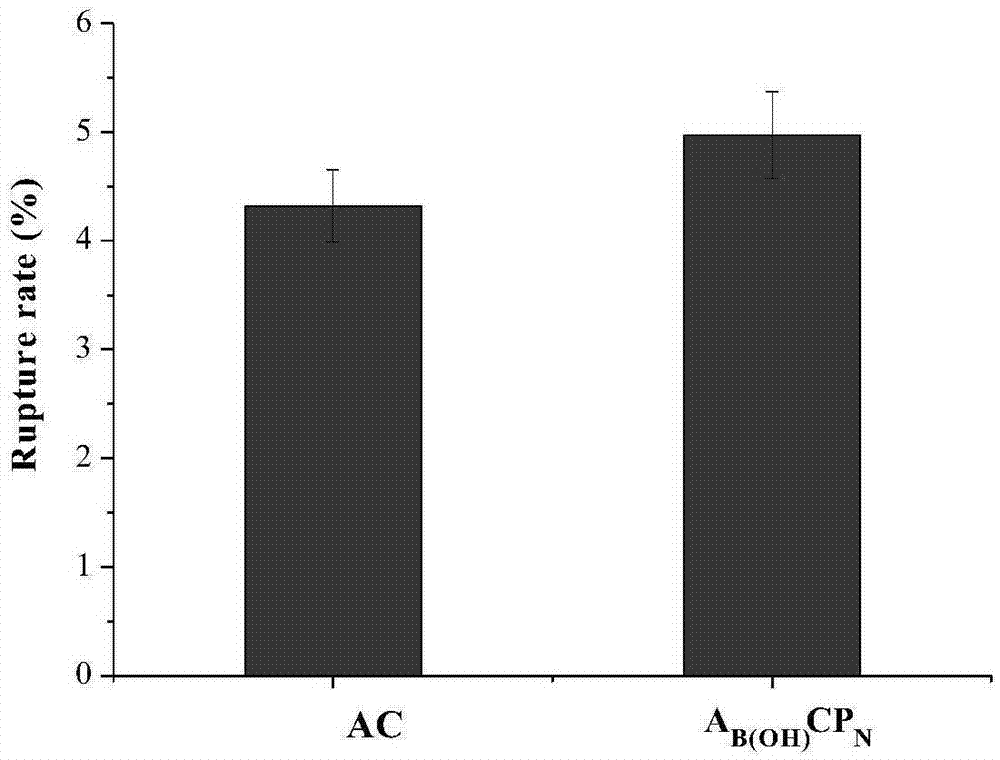
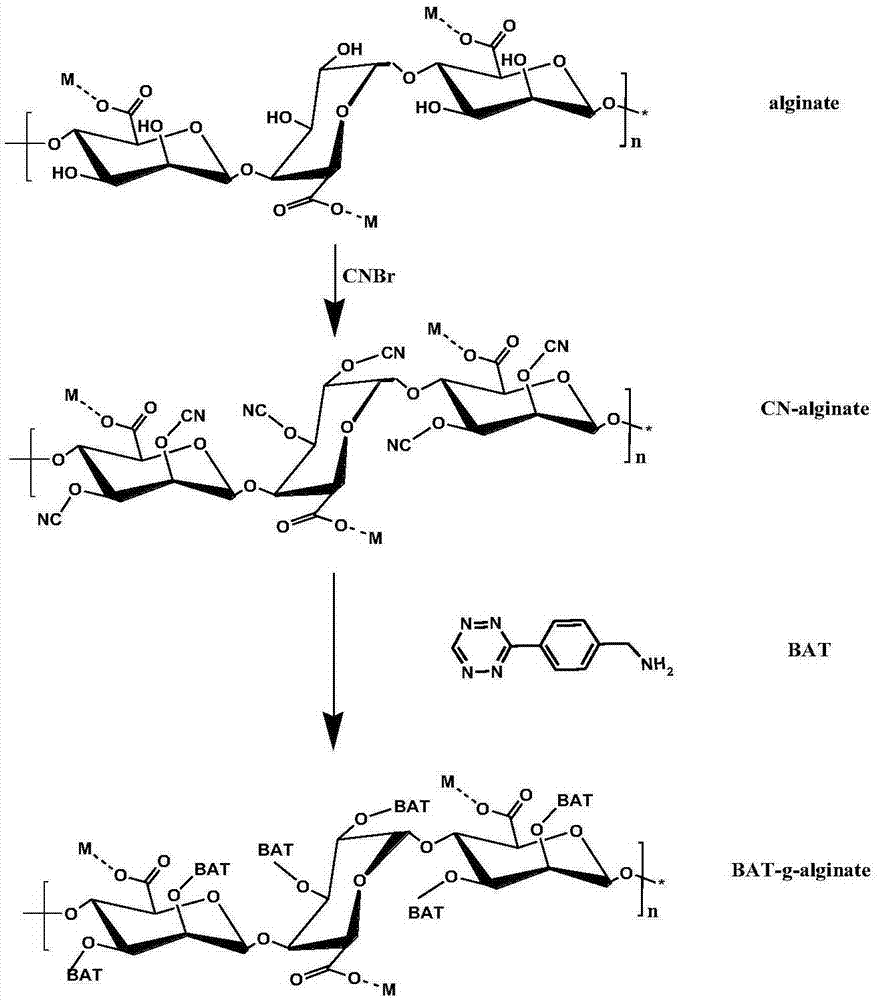
PEG in-situ covalently grafting modified alginate microcapsule, preparation and application thereof
A technology of alginate and covalent grafting, which is applied in the direction of microcapsules, capsule delivery, and medical preparations of non-active ingredients, etc. It can solve problems such as poor stability, small exclusion volume, and inability to achieve PEG grafting rate. To achieve the effect of improving stability and increasing graft density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) Preparation of alginate solution for covalent grafting of azide to hydroxyl sites: Dissolve sodium alginate in water to prepare a 1g / L sodium alginate solution, adjust the pH of the alginic acid solution to 10-11 between. Weigh cyanogen bromide (the molar amount of cyanogen bromide input: the molar amount of alginate monomer is 2:1), dissolve it, add it to the alginic acid solution, and constantly adjust the pH value of the alginic acid solution to keep it at 10-11 The activation time is 3 hours. Unreacted cyanogen bromide was removed by ultrafiltration, and 3-(4-aminomethylphenyl)-1,2,4,5-tetrazine (BAT) with a molecular weight of 187g / mol was added (the azide was put into the molar amount : The molar weight of the alginate monomer is 1.5:1), the reaction is stirred at room temperature for 2 days. The product was purified by ultrafiltration and freeze-dried to obtain sodium alginate with azide covalently modified hydroxyl site, and the grafting rate of azide compou...
Embodiment 2
[0065] 1) Prepare a solution of azide modified alginate: sodium alginate has a molecular weight of 200kDa, and the end of 3-(4-aminomethylphenyl)-1,2,4,5-tetrazine is activated by cyanogen bromide activation method The amino group is covalently bonded with the sodium alginate hydroxyl group, and the sodium alginate modification rate is 55%. The azide-modified sodium alginate is dissolved in physiological saline at a concentration of 50 g / L.
[0066] 2) Preparation of olefin modified PEG solution: PEG-NH 2 The molecular weight is 500Da, and PEG-NH is 2 The terminal amino group is covalently bonded with the terminal carboxyl group of 5-norbornene-2-carboxylic acid to obtain the olefin modified PEG, which is dissolved in physiological saline at a concentration of 25 g / L.
[0067] 3) Immerse the azide-modified calcium alginate gel microspheres embedded with porcine pancreatic islet cells in the PEG solution prepared in step 2). The volume ratio of the gel microspheres to the PEG solutio...
Embodiment 3
[0070] 1) Prepare a solution of azide modified alginate: sodium alginate has a molecular weight of 500kDa, and the terminal amino group of 3-aminomethyl-6methyl-1,2,4,5-tetrazine is combined with cyanogen bromide activation method Sodium alginate carboxyl groups are covalently bonded, and the modification rate of sodium alginate is 135%. Sodium alginate modified with azide is dissolved in physiological saline at a concentration of 10g / L.
[0071] 2) Preparation of α-polylysine solution: α-polylysine has a molecular weight of 20 kDa and is dissolved in physiological saline at a concentration of 5 g / L.
[0072] 3) Preparation of olefin modified PEG solution: PEG-NH 2 The molecular weight is 10kDa, and the PEG-NH is 2 The terminal amino group is covalently bonded with the terminal carboxyl group of 5-norbornene-2-carboxylic acid to obtain the olefin modified PEG, which is dissolved in physiological saline at a concentration of 5 g / L.
[0073] 4) Immerse the azide-modified calcium algina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com