Targeting ligand modified reduction-responsive magnetic nano carrier and preparation method thereof
A technology of magnetic nanocarriers and targeting ligands, which is applied in the direction of pharmaceutical formulations, preparations for in vivo tests, and medical preparations of non-active ingredients. Tumor targeting, simple preparation method, enhanced effect of tumor suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] With reference to the document J.Am.Chem.Soc.2004,126,273-279, iron acetylacetonate (0.7063g, 2mmol), 1,2-dodecanediol (2.0234g, 10mmol), oleic acid (l .6948g, 6mmol), oleylamine (1.605g, 6mmol), diphenyl ether (20mL) was stirred under argon flow at 110°C for 1h to remove water and oxygen, and the temperature was raised to 200°C (8°C per minute), React at this temperature for 2 hours, stop nitrogen, heat the temperature to 265°C, reflux for 30 minutes, wait for the reaction solution to cool to room temperature, pour the reaction solution into 40ml ethanol for precipitation, 11000r / min*30min, wash with absolute ethanol After three times, it was dispersed in 25 ml of chloroform for preservation.
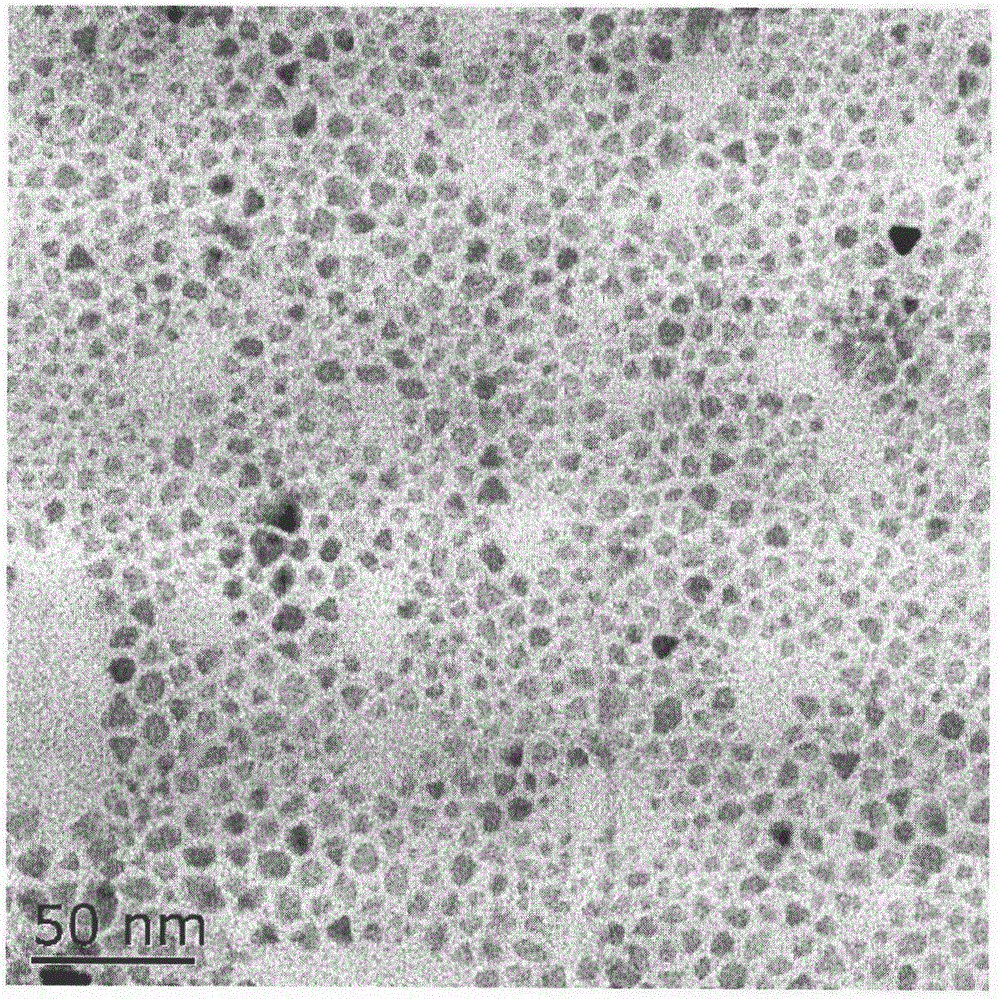
[0065] The morphology was observed by transmission electron microscopy (TEM), and the results were as follows: figure 2 , the particle shape is nearly spherical, and the particle size is below 10nm.
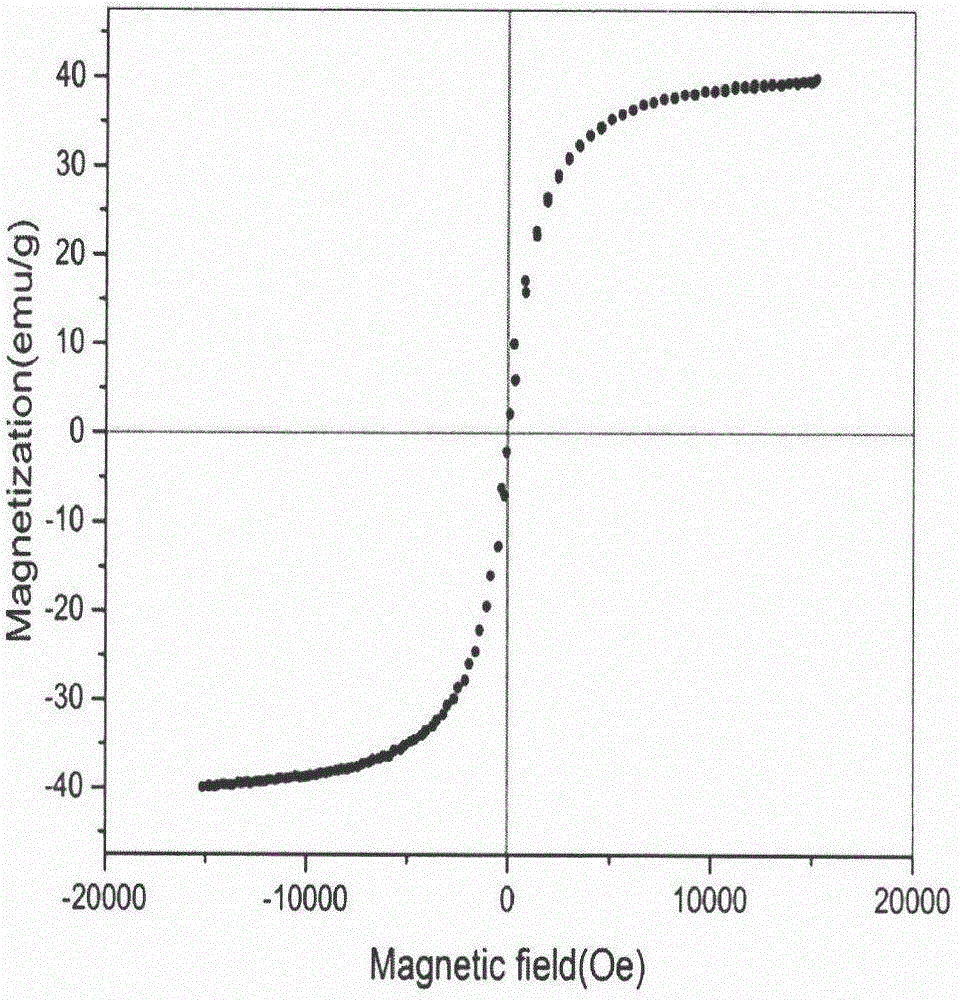
[0066] Its magnetic properties were characterized by a vibrating sample mag...
Embodiment 2
[0068] Example 2: Preparation of cetyl alcohol-modified chitosan oligosaccharide (CSO-SS-Hex) bonded with reduction-sensitive bond Linker
[0069] 1) Synthesis of a hydrophobic group bonded with a reduction-sensitive bond Linker:
[0070] 3,3'-dithiodipropionic acid (1.50g, 7.13mmol) and DCC (1.618g, 7.84mmol) were dissolved in 20ml of anhydrous DMF, stirred in an ice bath under argon protection for 30min, cetyl alcohol ( 1.729g, 7.13mmol) and DMAP (87mg, 0.713mmol) were added therein, continued to stir the reaction under ice bath for 1h, then transferred it to room temperature and stirred for 2h, added an equal volume of ethyl acetate to terminate the reaction, filtered to remove Dicyclohexylurea, the filtrate was extracted with ethyl acetate after adding a small amount of water, the ethyl acetate layers were combined, concentrated under reduced pressure, and recrystallized in 20ml of methanol / ethyl acetate=1 / 1 mixed solvent to obtain the product Hex-SS-COOH . That 1 H-NMR s...
Embodiment 3
[0077] Example 3: Preparation of cholesterol-modified chitosan oligosaccharides (CSO-SS-Chol) bonded with reduction-sensitive bond Linker
[0078] 1) Synthesis of a hydrophobic group bonded with a reduction-sensitive bond Linker:
[0079] 3,3'-dithiodipropionic acid (1.50g, 7.13mmol) and DCC (1.618g, 7.84mmol) were dissolved in 20ml of anhydrous THF, stirred in an ice bath under the protection of argon for 30min, and cholesterol (2.756g , 7.13mmol) and DMAP (87mg, 0.713mmol) were added therein, continued to stir the reaction under ice bath for 1h, then transferred it to room temperature and stirred for 12h, filtered to remove dicyclohexylurea, concentrated under reduced pressure, and dissolved in 20ml acetic acid The product Chol-SS-COOH was obtained by recrystallization in a mixed solvent of ethyl ester / n-hexane=1 / 1.
[0080] 2) Synthesis of hydrophobically modified chitosan oligosaccharides:
[0081] After dissolving 1 g of chitosan oligosaccharide with a molecular weight ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
| Saturation magnetic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com