Drug delivery vehicles for active customization of albumin corona and its application in pharmacy
An albumin and drug technology, applied in pharmaceutical formulations, liposome delivery, effective components of heterocyclic compounds, etc., can solve problems such as the inability to fully exert the design targeting function, and achieve the goal of overcoming the biological transmission barrier of tumors and small particle size. , the mild effect of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of the maleimide-modified carrier PLGA-Mal:
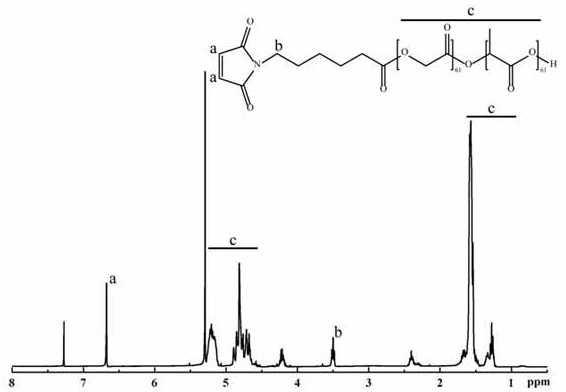
[0058] Put 6-aminocaproic acid and maleic anhydride in a three-necked flask, add glacial acetic acid to dissolve, reflux for 6 h in an oil bath at 135 °C, measure a little acetic anhydride, and add it dropwise to the reaction at a rate of 1 drop / second. solution, refluxed for 2 h, and separated with a silica gel column to obtain 6-maleimidocaproic acid. Weigh 6-maleimide caproic acid and add it to a 100 mL eggplant-shaped bottle, add oxalyl chloride and stir to dissolve the sample. Reflux for 2 h in an oil bath at 70°C with stirring to obtain 6-maleimide caproyl chloride. The reaction solution was spin-dried with a rotary evaporator to remove oxalyl chloride. The obtained 6-maleimide caproyl chloride was dissolved in redistilled dichloromethane, and magnetically stirred in an oil bath at 40 °C for 15 min. At the same time, PLGA was dissolved in redistilled dichloromethane, and the constant pressure dropping fu...
Embodiment 2
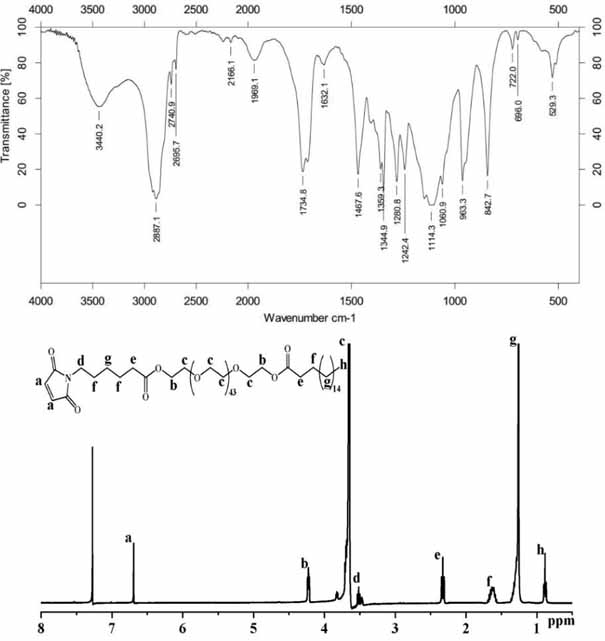
[0063] The preparation method of the maleimide-modified carrier SA-PEG-Mal is described in the following steps: respectively place 6-aminocaproic acid and maleic anhydride in a three-necked flask, add glacial acetic acid to dissolve, and place them in an oil bath at 135°C Reflux for 6 h, measure a little acetic anhydride, drop it into the reaction solution at a rate of 1 drop / second, reflux for 2 h, and use a silica gel column to separate to obtain 6-maleimidocaproic acid. Weigh 6-maleimidocaproic acid into a 100 mL eggplant-shaped bottle, add oxalyl chloride and stir to dissolve the sample. Reflux for 2 h in an oil bath at 70°C with stirring to obtain 6-maleimide caproyl chloride. The reaction solution was spin-dried with a rotary evaporator to remove oxalyl chloride. The obtained 6-maleimide caproyl chloride was dissolved in redistilled dichloromethane, and magnetically stirred in an oil bath at 40 °C for 15 min. At the same time, polyethylene glycol stearic acid was disso...
Embodiment 3
[0067] Preparation of Docetaxel-loaded Nanoparticles by Emulsion Solvent Evaporation
[0068] Weigh 1 mg of docetaxel or coumarin 6, dissolve in an appropriate amount of dichloromethane, add 20 mg of PLGA-Mal or PLGA prepared in Example 1, PLGA-PEG500-Mal, PLGA-PEG2000-Mal, PLGA- PEG2000, add 5 mL of deionized water containing 0.5% PVA, sonicate the probe at 300 W for 5 min, and remove uncoated drugs by centrifugation.
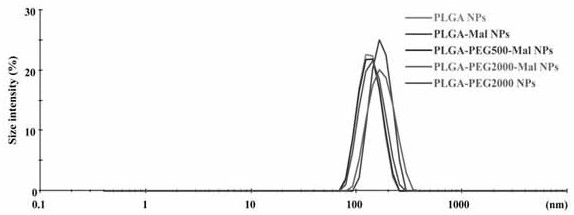
[0069] The nanoparticles prepared in Example 3 were measured by dynamic light scattering and transmission electron microscopy for particle size and shape. The result is as image 3 , the particle size of the nanoparticles is about 150 nm, and the particle size distribution is narrow; the transmission electron microscope shows that the drug-loaded nanoparticles are spherical with uniform particle size.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| aggregation | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com