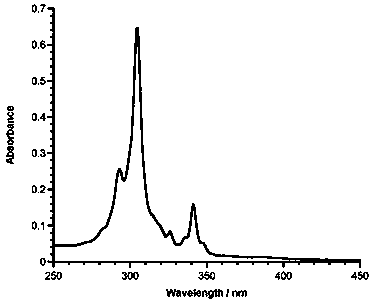
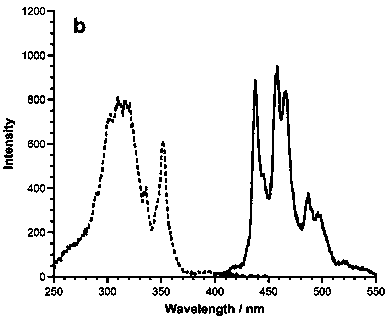
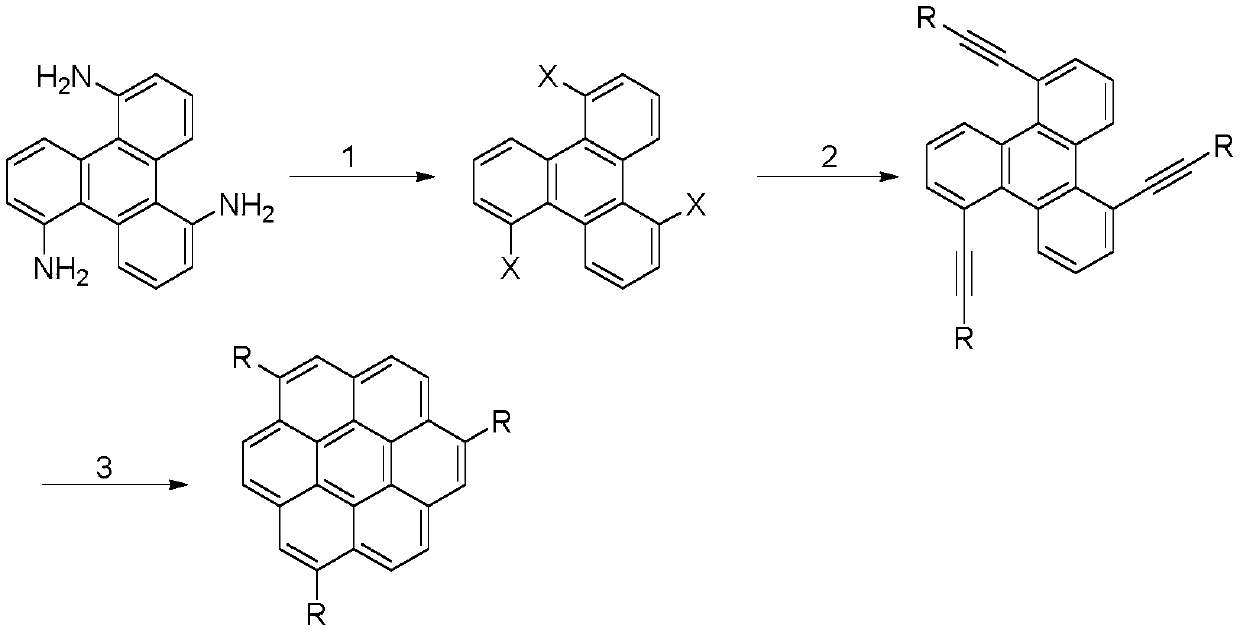
1,5,9-trisubstituted pine compound and its synthesis method
A compound and tri-substituted technology, applied in the field of polycyclic aromatic hydrocarbons and their preparation, can solve the problems of single structure, harsh conditions and long steps in the synthesis of pinames, and achieve the effects of short synthesis steps, little environmental pollution and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Taking the synthesis of 1,5,9-tri(p-methylphenyl)cone as an example, its structural formula is as follows:
[0032]
[0033] The raw materials used and their synthetic methods are:
[0034] 1. Synthesis of 1,5,9-triiodotriphenylene
[0035] Dissolve 2g of 1,5,9-triaminotriphenylene (refer to Chem.Commun.2016,52,537-540 for the preparation method) in hydrochloric acid solution (30mL water, 15mL hydrochloric acid), cool to 0°C in an ice-salt bath, and use a constant Add 20mL of 1M sodium nitrite aqueous solution dropwise into the pressure dropping funnel. After the dropwise addition, stir in an ice-salt bath for 1 h, then pour 30 mL of a 1.5 M potassium iodide aqueous solution, and react at 65°C until the foam disappears and a black solid is formed. After cooling and suction filtration, the solid was washed successively with water, sodium thiosulfate solution, water and ethanol again. The solid was dissolved in 200 mL of THF, and the filtrate was obtai...
Embodiment 2
[0041] Embodiment 2: Taking the synthesis of 1,5,9-tris(p-methoxyphenyl)cone as an example, its structural formula is as follows:
[0042]
[0043] The raw materials used and the synthesis method are:
[0044] In step 2 of this embodiment, p-methylphenylacetylene is replaced with equimolar p-methoxyphenylacetylene, and other steps of this step are the same as in Example 1. The eluent ratio in all steps is adjusted to 1:1, other steps are the same as in Example 1, a yellow solid 1,5,9-tris(p-methoxyphenyl)cone is prepared, and its yield is: 19 %, melting point: 141-143°C.
[0045] The characterization data of the resulting product are as follows:
[0046] 1 H NMR (500MHz, CDCl 3 ): δ9.01(d, J=8.6Hz, 3H), 8.85(d, J=8.8Hz, 3H), 8.83(s, 3H), 7.85(d, J=8.3Hz, 6H), 7.24(d ,J=8.3,6H),4.02(s,9H); 13 C NMR (125MHz, CDCl 3 ): δ159.3, 138.8, 133.9, 132.0, 128.4, 127.4, 126.9, 126.1, 125.0, 123.3, 122.2, 114.1, 55.6; HRMS (DART) calcd for C 45 h 31 o 3 [M+H] + 619.2268,found...
Embodiment 3
[0047] Embodiment 3: Taking the synthesis of 1,5,9-tributylcone as an example, its structural formula is as follows:
[0048]
[0049] The raw materials used and the synthesis method are:
[0050] In step 2 of this example, 1g of 1,5,9-triiodotriphenylene, 57mg of bis(triphenylphosphine)palladium dichloride, 12.6mg of cuprous iodide, 488mg of 1-hexyne were added to the flask, 50 mL of triethylamine, the molar ratio of 1,5,9-triiodotriphenylene, bis(triphenylphosphine)palladium dichloride, cuprous iodide and 1-hexyne is 1:0.05:0.04:3.6. Under the protection of inert gas, react at 50°C for 12h. Then the solvent was evaporated, and the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 10:1 was used as an eluent to separate by silica gel column chromatography to obtain light yellow oily liquid 1,5,9-trihexynetriphenylene, which produced Rate: 51%.
[0051] In step 3 of this example, 1,5,9-hexynyltriphenylene and 1,8-diazabicycloundec-7-ene were adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com