Respiratory syncytial virus vaccine, and preparation method and application thereof
A technology of syncytial virus and respiratory tract, which is applied in the field of respiratory syncytial virus vaccine and its preparation, can solve the problems of low neutralizing antibody level, unguaranteed safety, and the advent of RSV vaccine, so as to inhibit inflammation-related pathological reactions and be easy to promote , effective and specific inflammation-related pathological responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The preparation method of inactivated vaccine FI-RSV: 10 7 TCID 50 RSV virus (US ATCC, catalog no. VR-26 TM ) was reacted with formaldehyde at 37°C for 72 hours, and purified by high-speed centrifugation at 50,000g for 1 hour.
[0058] Cyclosporin A (CSA) is a product of Taiwan Listed Chemical Pharmaceutical Co., Ltd., with a product catalog number of H10940111.
[0059] Vector pET28a(+) is a product of Novagen Company, catalog number 69864-3.
[0060] Escherichia coli BL21(DE3) is a product of Tiangen Biochemical Technology (Beijing) Co., Ltd., catalog number CB105-02.
[0061] 1640 medium is the product of Maichen Technology Co., Ltd., product number CM10040.
[0062] Goat anti-RSV polyclonal antiserum is a product of Meridian Company in the United States, the product catalog number is B656860G.
[0063] HBV vaccine is a product of Huada Pharmaceutical Co., Ltd.
Embodiment 1
[0064] Embodiment 1, the preparation of respiratory syncytial virus (RSV) vaccine
[0065] 1. Acquisition of G protein
[0066] 1. Acquisition of the gene encoding the G protein
[0067] The coding gene sequence of the respiratory syncytial virus G protein of the present invention is shown in sequence 1 in the sequence listing, and the amino acid sequence of the respiratory syncytial virus G protein is shown in sequence 2 in the sequence listing.
[0068] 2. Construction of expression vector pET28a-noG
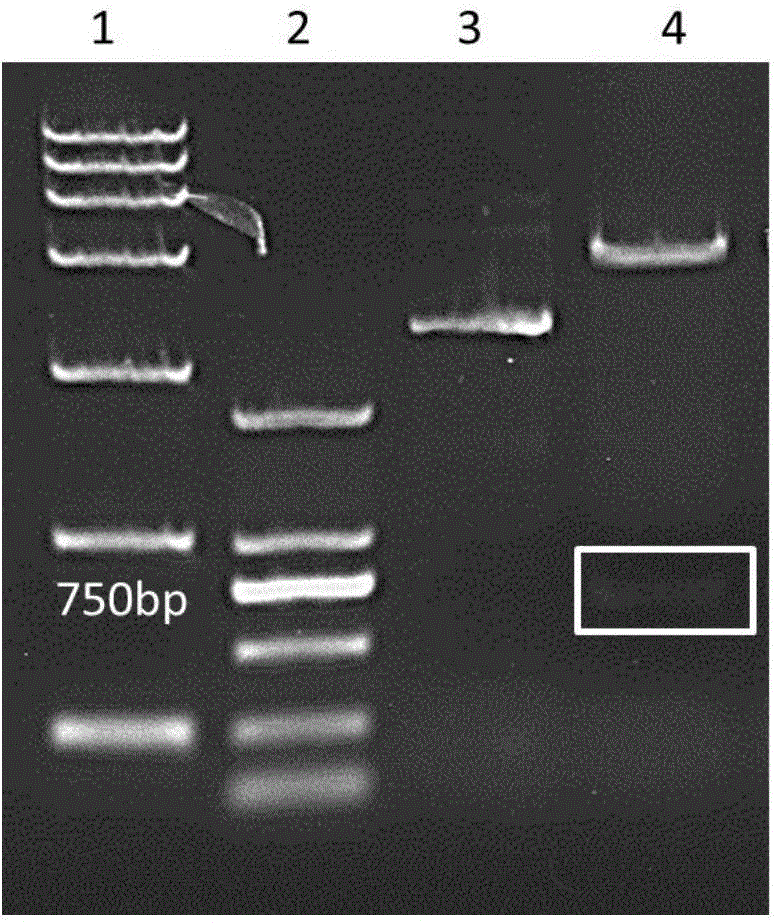
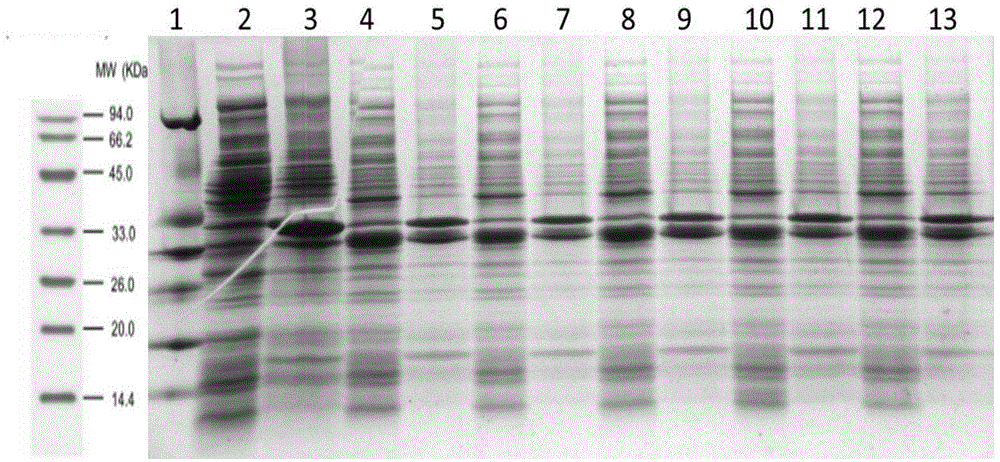
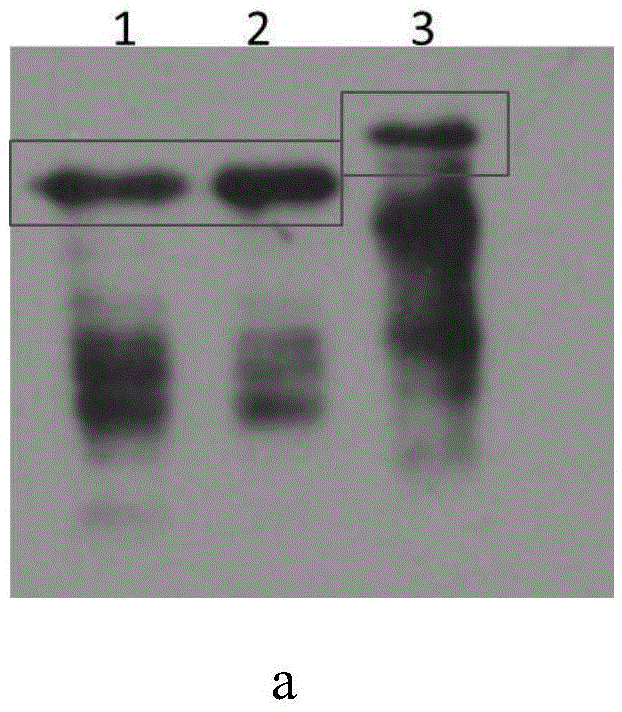
[0069] Synthesize the coding gene sequence of the respiratory syncytial virus G protein in step 1 by the method of whole gene synthesis, carry out double enzymes to the coding gene sequence of the respiratory syncytial virus G protein and the carrier pET28a (+) with restriction endonuclease NcoI and XhoI cutting and ligation to obtain a recombinant vector, which was named pET28a-noG. The results of enzyme digestion and identification of the pET28a-noG vector are as follows:...
Embodiment 2
[0101] Embodiment 2, detection of antibody level after respiratory syncytial virus (RSV) vaccine immunization mice
[0102] 1. Vaccine immunization mice in each group
[0103] Experimental materials: 6-8 weeks old female BALB / c mice, purchased from the Experimental Animal Department of Fudan University, clean grade, divided into 9 groups, 5 mice in each group. During the experiment, sterile water and food were used for feeding, and the photoperiod was 12 hours.
[0104] Experimental vaccine is divided into the respiratory syncytial virus (RSV) vaccine group (G+CSA), subunit vaccine group (G), PBS group (negative control), FI-RSV group (positive control) of the four preparations of embodiment 1. Respiratory syncytial virus (RSV) vaccine group is divided into (1ugCSA+1ug G protein) group, (2ug CSA+10ug G protein) group, (4ug CSA+10ug G protein) group, (6ug CSA+10ug G protein) group according to CSA and G protein quality difference again in respiratory syncytial virus (RSV) vacc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com