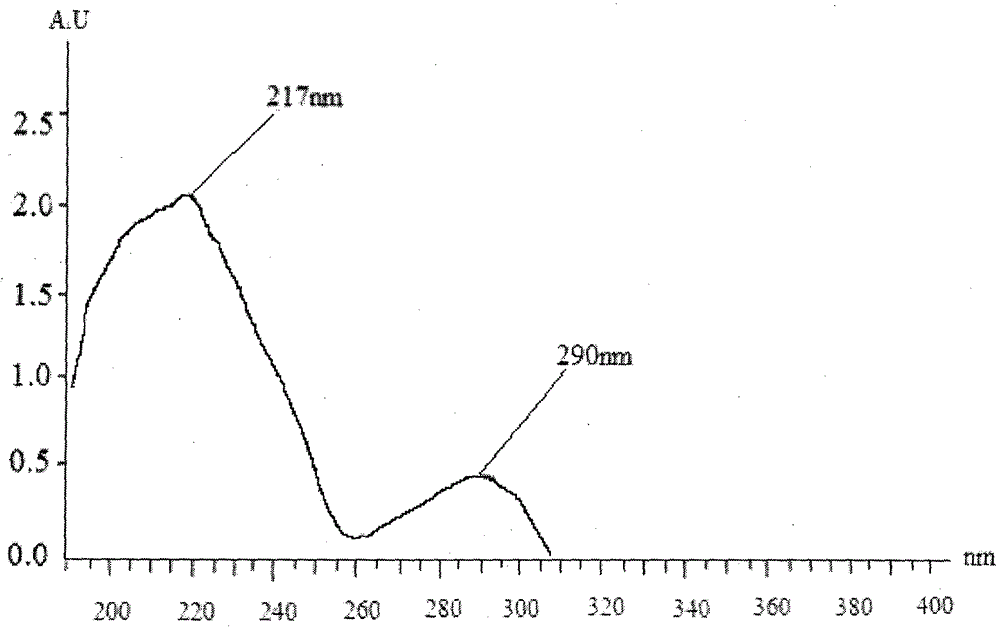
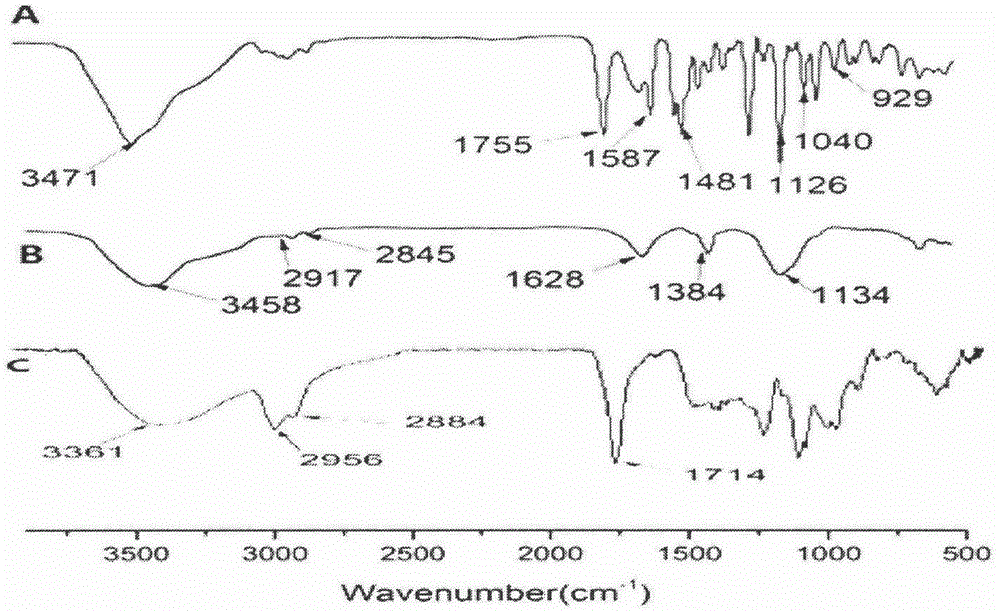
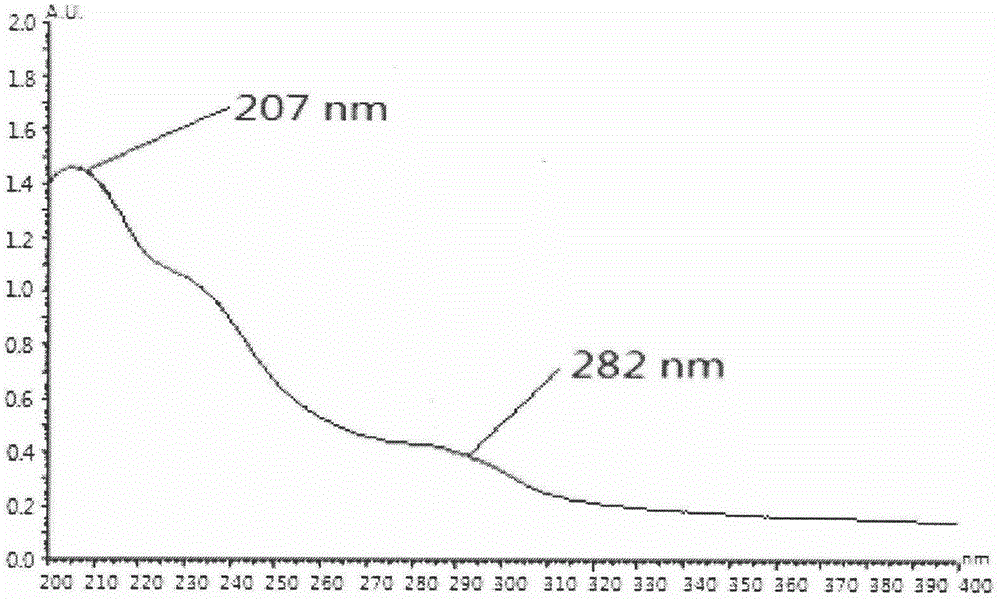
Preparation method and application of podophyllotoxin transdermal drug delivery preparation
A podophyllotoxin and transdermal drug delivery technology, applied in antiviral agents, pharmaceutical formulations, skin diseases, etc., to achieve good clinical application value, convenient use, and easy to meet the preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation and Characterization of PPT-CNTs-COOH
[0035] Precisely weigh 5 mg of podophyllotoxin raw material drug and ultrasonically dissolve it in 10 mL of absolute ethanol for later use; accurately weigh 50 mg of multi-walled carboxylated carbon nanotubes (CNTs-COOH) and place it in a ball mill jar; add podophyllotoxin in absolute ethanol three times Solution, 580rpm·min under the protection of liquid nitrogen 1 Ball mill three times, 6 minutes each time; collect the ball-milled mixture in a stoppered Erlenmeyer flask (protected from light), and let it stand for 12 hours; filter the unadsorbed medicinal solution with a 0.45 μm organic filter membrane, and put the filter cake into a petri dish After evaporating absolute ethanol, add 10mL of purified water, place in a centrifuge tube and centrifuge for 1h (12000rpm·min 1); purify the centrifuged supernatant through a Sephadex column, use water as the eluent, and freeze-dry the eluent to obtain the PPT-CNT...
Embodiment 2
[0039] Embodiment 2: Determination of PPT-CNTs-COOH content
[0040] Chromatographic conditions: Column: EcosilC 18 column; mobile phase (water-methanol: 40-60); flow rate (1mL min -1 ); detection wavelength (292nm); column temperature (room temperature); injection volume (10μL).
[0041] Preparation of reference substance solution: Accurately weigh 1.2mg of PPT reference substance in a 25mL measuring bottle, dissolve in methanol and dilute to the mark to prepare 44.88μg·mL -1 The reference substance solution was obtained by passing through a 0.45 μm microporous membrane.
[0042] Linearity and range: Precisely pipette 2mL, 4mL, 6mL, 8mL, 10mL to 10mL of the reference substance solution into a 10mL measuring bottle, prepare a series of standard solution, and inject 10μL to measure the peak area. Carry out linear regression with the quality of the drug injected as the abscissa and the peak area as the ordinate, and the linear equation is: y=620.39x-811.20, the correlation co...
Embodiment 3
[0045] Example 3: Three batches of samples prepared by expanded production of PPT-CNTs-COOH and their content determination results
[0046] Precisely weigh 50g of podophyllotoxin raw material drug and ultrasonically dissolve it in 1000mL of absolute ethanol for later use; accurately weigh 500mg of multi-walled carboxylated carbon nanotubes (CNTs-COOH) and place it in a ball mill jar; add podophyllotoxin in absolute ethanol solution, 580rpm·min under liquid nitrogen protection 1 Ball mill three times, 6 minutes each time; collect the ball-milled mixture in a stoppered Erlenmeyer flask (protected from light), and let it stand for 12 hours; filter the unadsorbed medicinal solution with a 0.45 μm organic filter membrane, and put the filter cake into a tray , after evaporating the ethanol to dryness, put it in a beaker, add 1000mL of purified water, stir and dissolve, put it in a centrifuge tube and centrifuge for 1h (12000rpm·min 1 ); purify the centrifuged supernatant through a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Skin penetration rate | aaaaa | aaaaa |
| Skin penetration rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com