Application of 2-hydroxypropyl-β-cyclodextrin in preparation of medicine for treating X-linked adrenoleukodystrophy
A cyclodextrin, adrenal brain technology, applied in the field of biomedicine, can solve the problems of staying, infeasible, unable to prevent demyelination, etc., and achieve the effect of reducing the level of VLCFA and alleviating abnormal behavioral symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
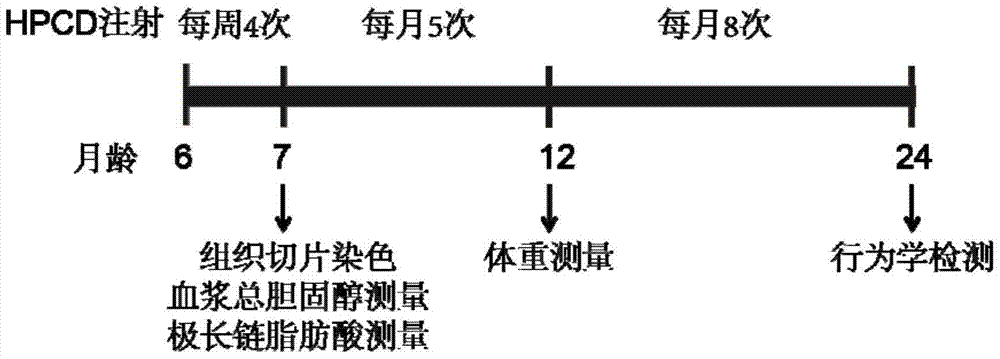
[0021] Example 1 HPCD injection X-ALD mouse procedure
[0022] The materials used in this example: 2-hydroxypropyl-β-cyclodextrin (2-hydroxypropyl-β-cyclodextrin) HPCD was purchased from Cyclodextrin Technologies Development, Inc., and ABCD1 knockout mice were purchased from Jackson Laboratory.
[0023] according to figure 1 The procedure shown is for wild-type (WT) and X-ALD model mice (ABCD1 knockout mice, ABCD1 - / - Mice) were injected with HPCD on the back of the neck. The injection dose was 4000mg HPCD per kg of mouse body weight. The details were as follows: 20% HPCD (W / V) was prepared with 0.9% saline, and 6-month to 7-month-old mice were injected 4 weekly 20% HPCD, the control group was injected with the same volume of normal saline. Filipin staining of cells, Filipin staining of adrenal and cerebellar tissue sections, and measurement of plasma total cholesterol and very long-chain fatty acids were performed at 7 months of age; 7 months to 12 months of age The mice were inje...
Embodiment 2
[0024] Example 2 HPCD relieves cholesterol accumulation in X-ALD mouse cells
[0025] Material used in this example: Filipin was purchased from Sigma.
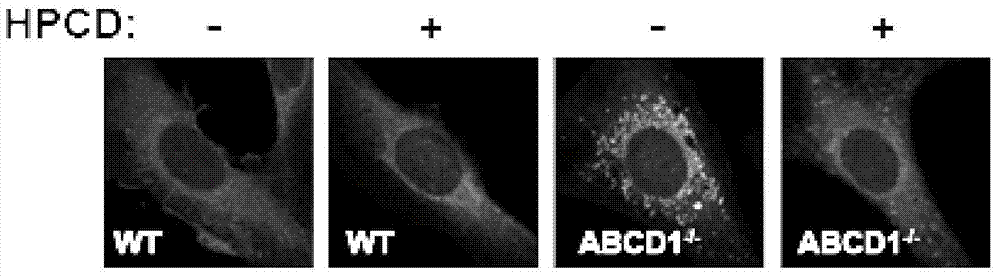
[0026] In order to test whether HPCD can improve the symptoms of cholesterol accumulation at the cell level of X-ALD mice, the 7-month-old WT and ABCD1 injected with saline and HPCD in Example 1 were taken. - / - Mice, separated and cultured their tail tip fibroblasts for observation by Filipin staining.
[0027] Filipin staining: the isolated and cultured tail tip fibroblasts are fixed with 4% paraformaldehyde for 30 min at room temperature; washed twice with PBS; dilute the ethanol-dissolved Filipin mother solution (5 mg / mL) with PBS containing 10% fetal bovine serum (FBS) to The final concentration is 50μg / mL, and incubate at room temperature for 30min in the dark; wash with PBS three times and wash with deionized water twice; mount the slides, dry overnight, observe with laser confocal microscope, and store at -20℃.
[0028] Filipin...
Embodiment 3
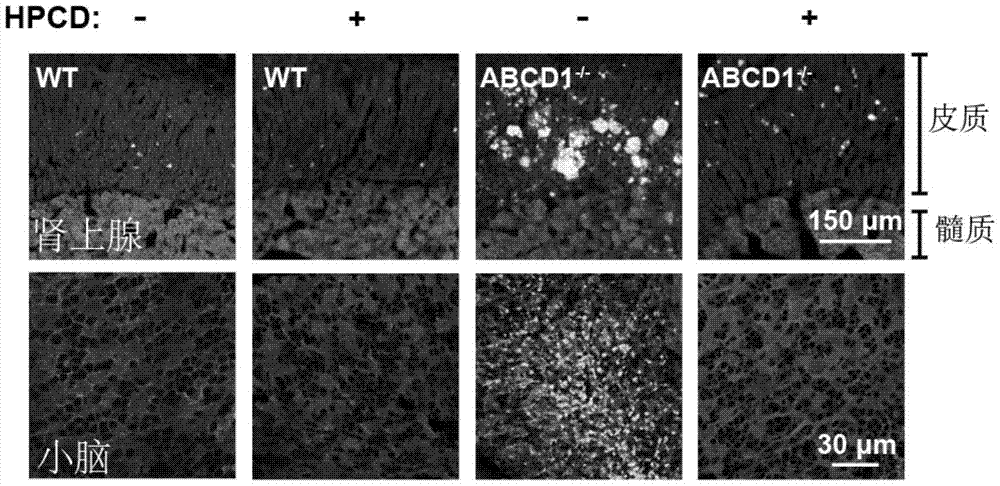
[0029] Example 3 HPCD relieves the accumulation of cholesterol in the tissues of X-ALD mice, reduces plasma total cholesterol and very long chain fatty acids (VLCFA)
[0030] To test whether HPCD can improve the symptoms of cholesterol accumulation in the tissues of X-ALD mice, reduce plasma total cholesterol and very long chain fatty acids (VLCFA). Take 7-month-old WT and ABCD1 injected with saline and HPCD in Example 1. - / - In mice, the adrenal glands and cerebellum were separated for frozen section and Filipin staining, and the plasma total cholesterol and very long-chain fatty acids were measured.
[0031] (1) Tissue section staining: cut the thoracic cavity of the mouse after anesthesia, insert a 20mL syringe connected to the scalp needle through the left ventricle, and cut a small mouth at the right atrial appendage with scissors, push in 20mL saline, and quickly change to 20mL after pushing. 4% paraformaldehyde; take out the adrenal glands and cerebellum of the required tiss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com