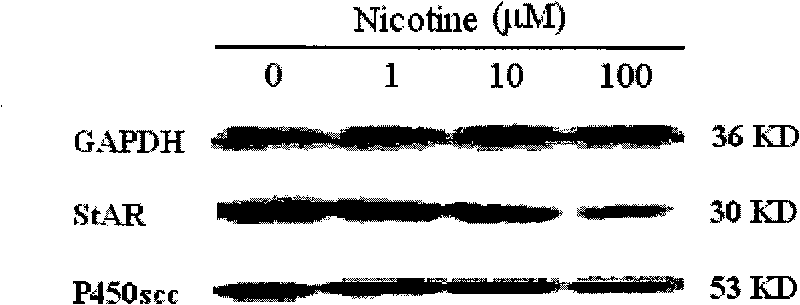Application of embryo adrenal StAR genes in assessing developmental toxicity of chemicals
A technology of adrenal gland and chemicals, which is applied in the application field of fetal adrenal gland StAR gene in the evaluation of chemical developmental toxicity, which can solve the problems of insensitivity of detection indicators, limitation of developmental toxicity evaluation work efficiency, and long experiment time, so as to promote the development of embryonic tissue. Developmental and functional maturation, reduced number of experimental animals, wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Effects of Nicotine and Cotriptyline on StAR and P450scc Expression in Human Fetal Adrenal Gland
[0028] 1. Reagent source
[0029] Nicotine and cotinine were purchased from Sigma Company of the United States; Trizol Reagent and HiBindTM PCR Product Recovery Kit were produced by Omega Company of the United States; primers used in the experiment were synthesized by Shanghai Sangon Bioengineering Technology Co., Ltd.; reverse transcription kits were purchased from Dalian Bao Bioengineering Co., Ltd. Co., Ltd.; the real-time quantitative RT-PCR kit was purchased from Takara Company in Japan; the Sybr Green I dye was purchased from Shanghai Jierui Bioengineering Co., Ltd.; the DMEM / F-12 medium was produced by Gibico Company in the United States; collagenase type I was purchased from Invitrogen in the United States Produced by the company; DNase I (DNase I) was purchased from Beijing Huamei Bioengineering Company; fetal bovine serum was purchased from Hangzhou Sij...
Embodiment 2
[0046] Example 2 Effects of nicotine, caffeine and ethanol on the expression of StAR and P450scc in human fetal adrenal gland NCI-H295A cells
[0047] 1. Reagent source
[0048] Nicotine and caffeine were purchased from Sigma, USA; primers used in the experiment were synthesized by Integrated DNA Technologies; RNA extraction kit (RNeasy Mini Kit) was purchased from Qiagen; reverse transcription kit (SuperScript TM II RNase H Reverse Transcriptase Kit) and PCR kit were purchased from Invitrogen; RPMI1640 medium and fetal bovine serum were purchased from Invitrogen; insulin, transferrin and selenite (IST) were purchased from sigma; BCA protein quantification reagent The box was purchased from Biorad; the mouse anti-StAR antibody was purchased from abcam; the β-tubulin antibody was from Santa Cruz Biotechnology; the Alexa Fluor 680 goat anti-mouse fluorescent secondary antibody was purchased from Rockland Immunochemicals.
[0049] 1. Experimental method
[0050] (1) Gene sourc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com