Application of taurochenodeoxycholic acid in preventing and treating adverse reactions of glucocorticoid medicaments
A technology of taurochenodeoxycholic acid and glucocorticoid, which is applied in the directions of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve the problem of wound healing, adverse effects of drug users' recovery, rebound phenomenon, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
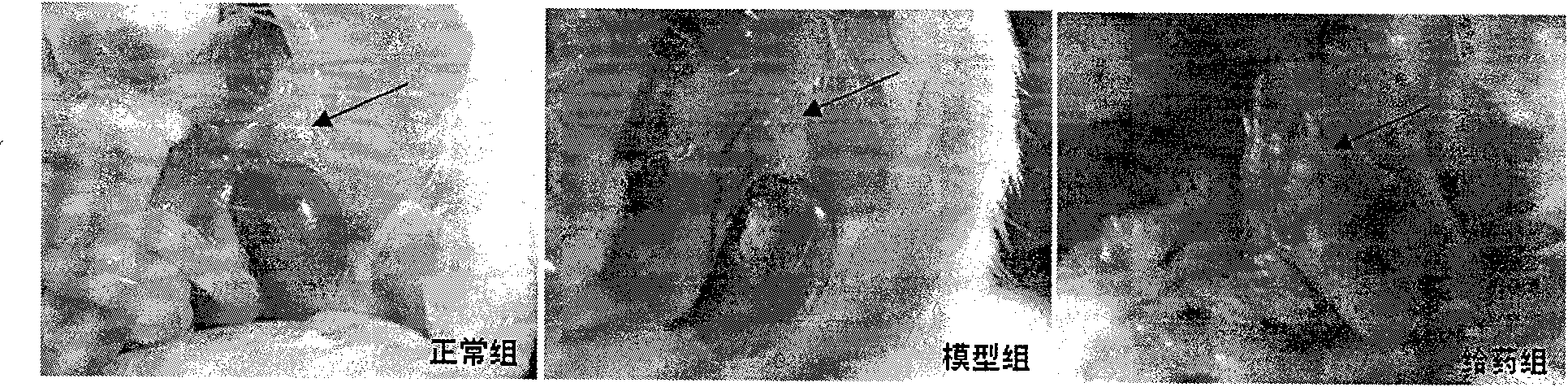
[0030] During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of adrenal suppression caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. The mice were dissected in the normal group, the model group and the middle-dose administration group, especially the adrenal gland was observed, and the results of the anatomical changes of the adrenal gland were found in the attached figure 1 And attached Figure 5 shown. It can be seen that, compared with the administration group, the model group showed symptoms of congestion...
Embodiment 2
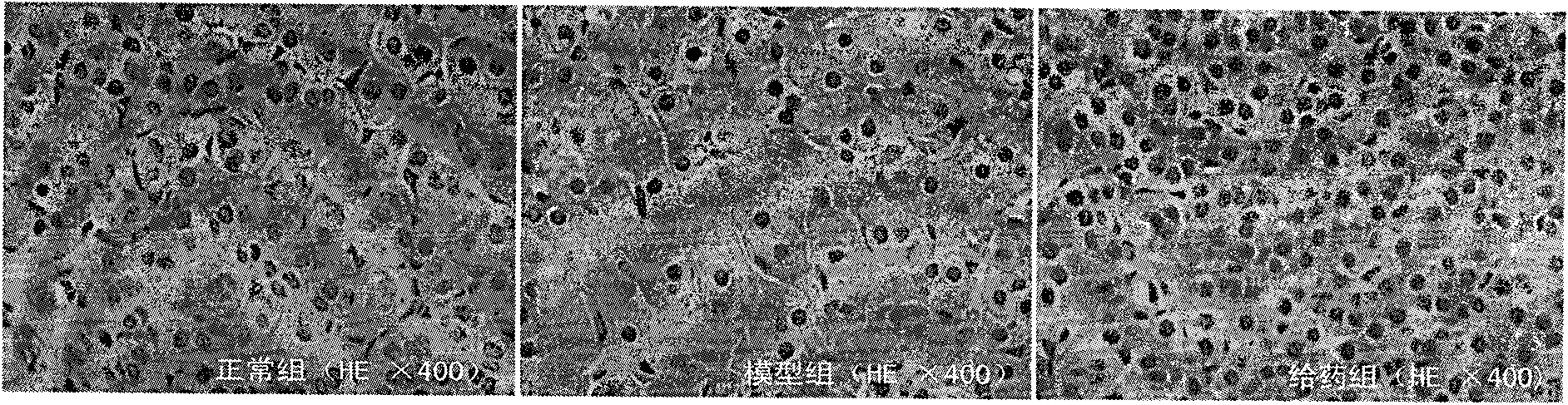
[0032]During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of adrenal suppression caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. HE staining of paraffin sections was used to evaluate the influence of TCDCA on the pathological changes of the adrenal gland, and the results are shown in the appendix figure 2 And attached Figure 6 shown. In the model group, the epithelial cells of the adrenal ducts had granular degeneration, decreased in number, and congestion of small blood vessels in the interstitium of th...
Embodiment 3
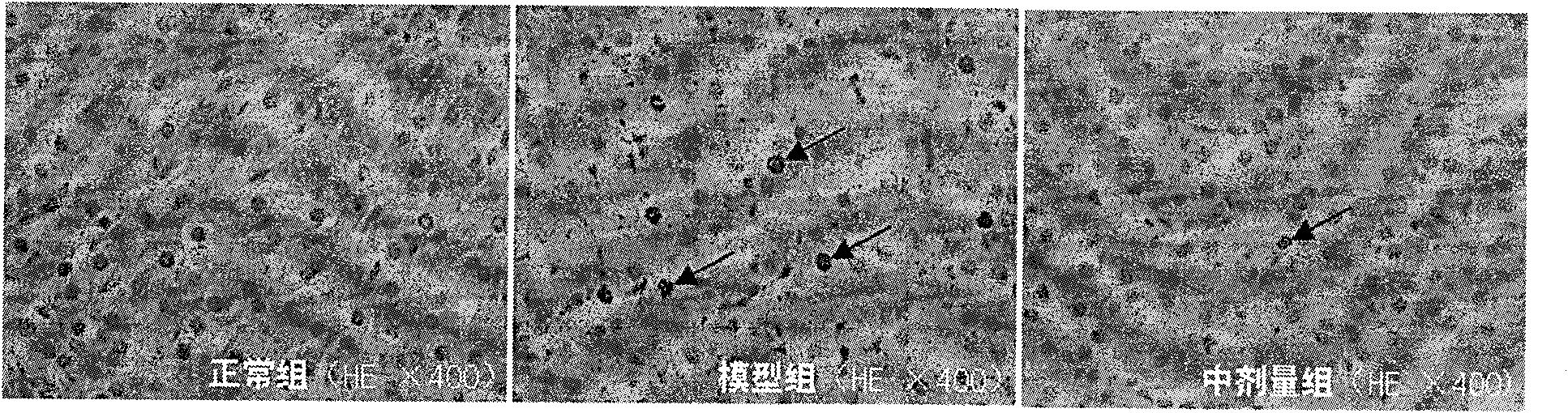
[0034] During the experiment, they were divided into normal group, model group and drug administration group, each with 10 mice. The normal group was only fed with distilled water; the model group was injected intraperitoneally with 80 mg / kg dexamethasone for 7 consecutive days to prepare the mouse model of adrenal suppression caused by glucocorticoids; the administration group was prepared in the model After completion of the administration, the dose of TCDCA in the administration group was 0.1 g / kg, and the route of administration was intragastric administration, once a day, for 7 consecutive days. TUNEL method was used to detect the effect of TCDCA on the apoptosis of adrenal gland cells. The tissue sections were observed and photographed under a 400-fold optical microscope. Dark brown cells were apoptotic cells, and light blue cells were normal cells. Three consecutive fields of view were selected in the adrenal cortex fascicularis, and the number of stained cells and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com