A kind of anti-inflammatory, analgesic medicine and preparation thereof
A mixture and regulator technology, applied in the field of medicine, can solve the problems of poor drug compliance and safety, need to improve the safety of use, slow incidence of phlebitis, etc., to meet the needs of industrial production, facilitate clinical medication, and be suitable for large batches. production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: Preparation example of compound of the present invention
[0055] Add 1mol of 2-[(2,6-dichlorophenyl)amino]phenylacetic acid sodium into the reaction vessel, add 1L of N,N-dimethylacetamide to dissolve, add 1.1mol of sodium carbonate as a catalyst, and After stirring at low temperature for 2 hours, 1.2 mol of 1-bromoethyl acetate was added dropwise, and the temperature was raised to 40° C. after 10 minutes of reaction at room temperature, and cooled to room temperature after 4 hours of reaction; a mixed solvent of 3L ethyl acetate and water (acetic acid Ethyl ester: water 1:1), separate the organic phase, wash with 3L of water, wash with 3L aqueous sodium thiosulfate (pH=10), and finally wash with 3L saturated brine; the organic phase is obtained by rotary evaporation of the organic solvent The crude product was vacuum-dried for 10 hours to remove the residual organic solvent to obtain 272.46 g of viscous oily substance, which was the target product diclof...
Embodiment 2
[0059] Embodiment 2: Preparation example of compound of the present invention
[0060]Add 1mol of sodium 2-[(2,6-dichlorophenyl)amino]phenylacetate to the reaction vessel, add 0.5L of N,N-dimethylformamide and 0.3L of acetone to dissolve, add 1.2mol of carbonic acid Potassium was used as a catalyst. After stirring at room temperature for 4 hours, 1.5 mol of 1-bromoethyl acetate was added dropwise. After 30 minutes of reaction at room temperature, the temperature was raised to 80 ° C. After 8 hours of reaction, it was cooled to room temperature; 5L of ethyl acetate and Mixed solvent of water (ethyl acetate: water 1.5:1), separate the organic phase, wash with 5L of water, wash with 5L of sodium thiosulfate aqueous solution (pH=11), and finally wash with 5L of saturated brine; the organic phase is rotated After evaporating the organic solvent, a viscous oily crude product was obtained. After the crude product was vacuum-dried for 24 hours to remove the organic solvent residue, 26...
Embodiment 3
[0061] Embodiment 3: Preparation example of compound of the present invention
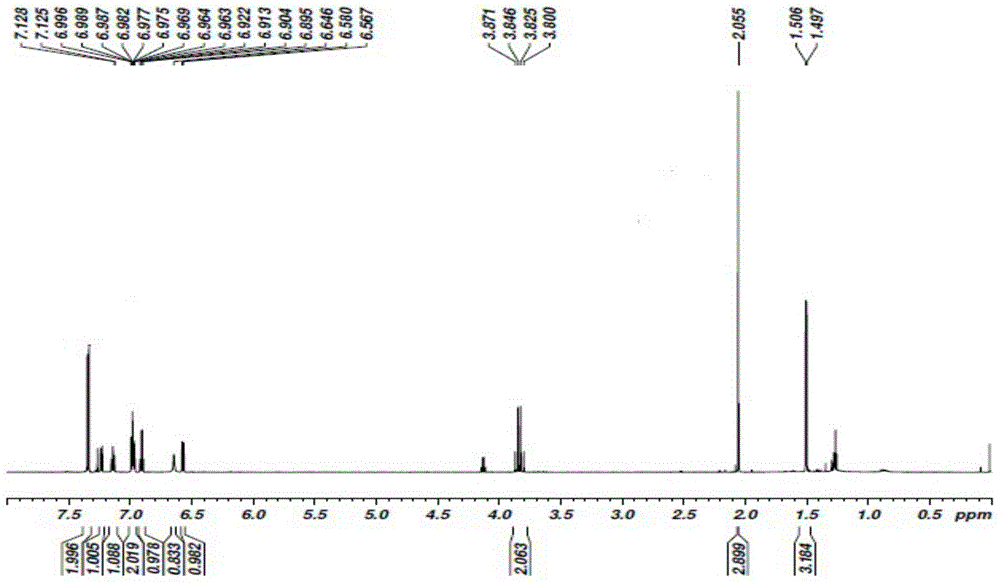
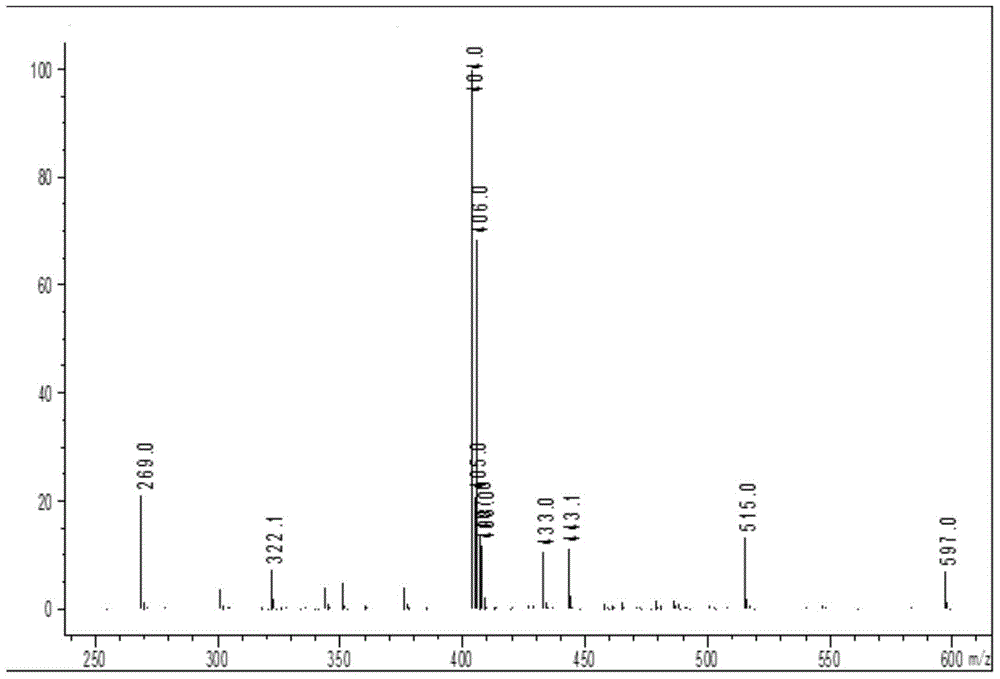
[0062] Add 1mol of sodium 2-[(2,6-dichlorophenyl)amino]phenylacetate to the reaction vessel, add 0.5L of N,N-dimethylacetamide and 0.3L of acetone to dissolve, add 1mol of sodium carbonate As a catalyst, after stirring at room temperature for 1 hour, add 1.1 mol of 1-bromoethyl acetate dropwise, react at room temperature for 20 minutes, then heat up to 50°C, and cool to room temperature after reacting for 5 hours; add 4L of ethyl acetate and water mixed solvent (ethyl acetate:water 1.2:1), separate the organic phase, wash with 4L of water, wash with 4L aqueous sodium thiosulfate (pH=10.2), and finally wash with 4L saturated brine; the organic phase is rotary evaporated After the organic solvent was removed, a viscous oily crude product was obtained. After the crude product was vacuum-dried for 8 hours to remove the residual organic solvent, 268.02 g of a viscous oily substance was obtained. 1 HNMR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com