BaFeO3-theta-base B-site Bi2O3 doping solid oxide fuel cell cathode material as well as preparation method and application thereof
A fuel cell cathode and solid oxide technology, applied in solid electrolyte fuel cells, battery electrodes, circuits, etc., can solve unconsidered problems and achieve improved structural stability, good oxygen reduction catalytic activity, and good thermal expansion coefficient matching Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
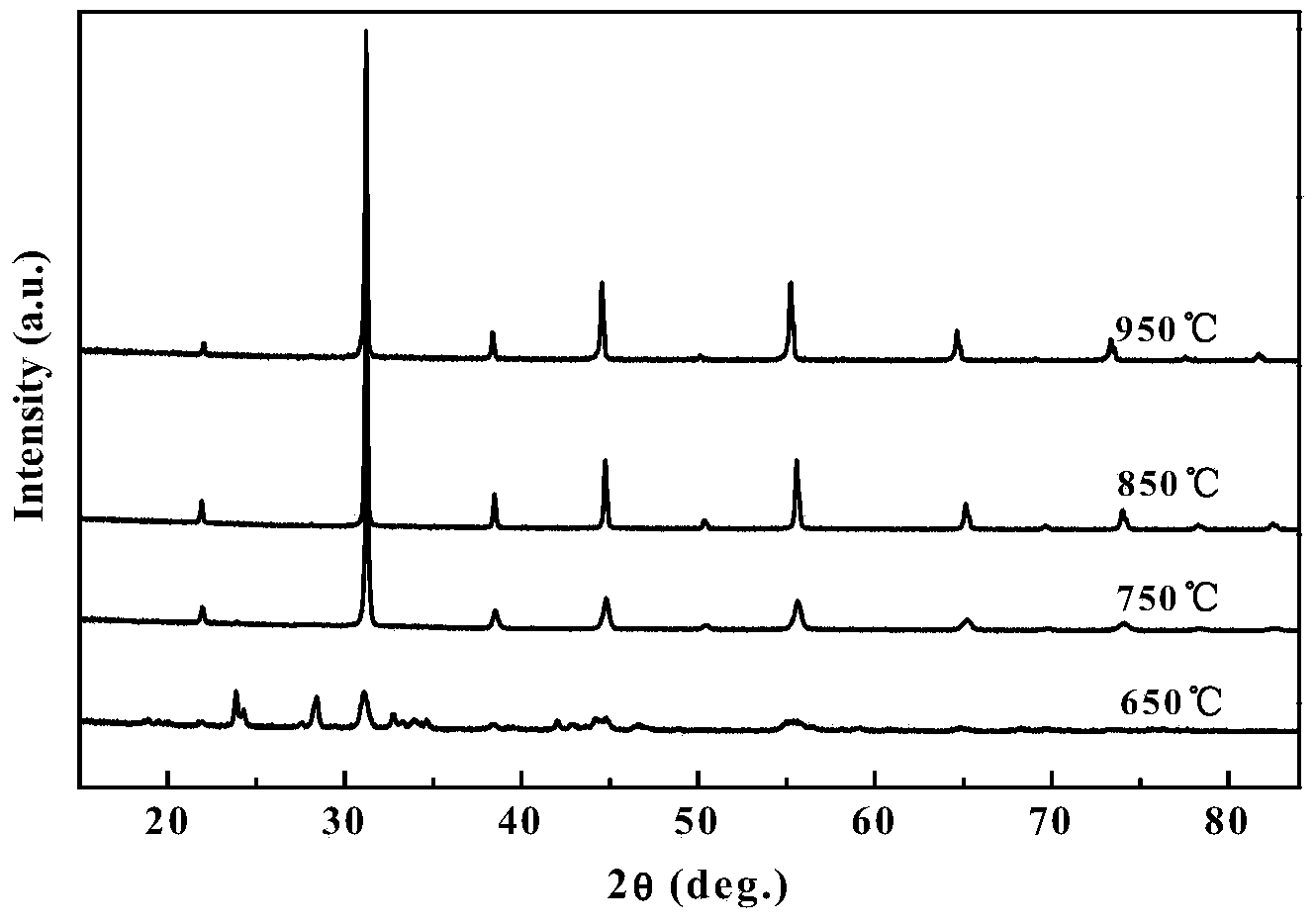
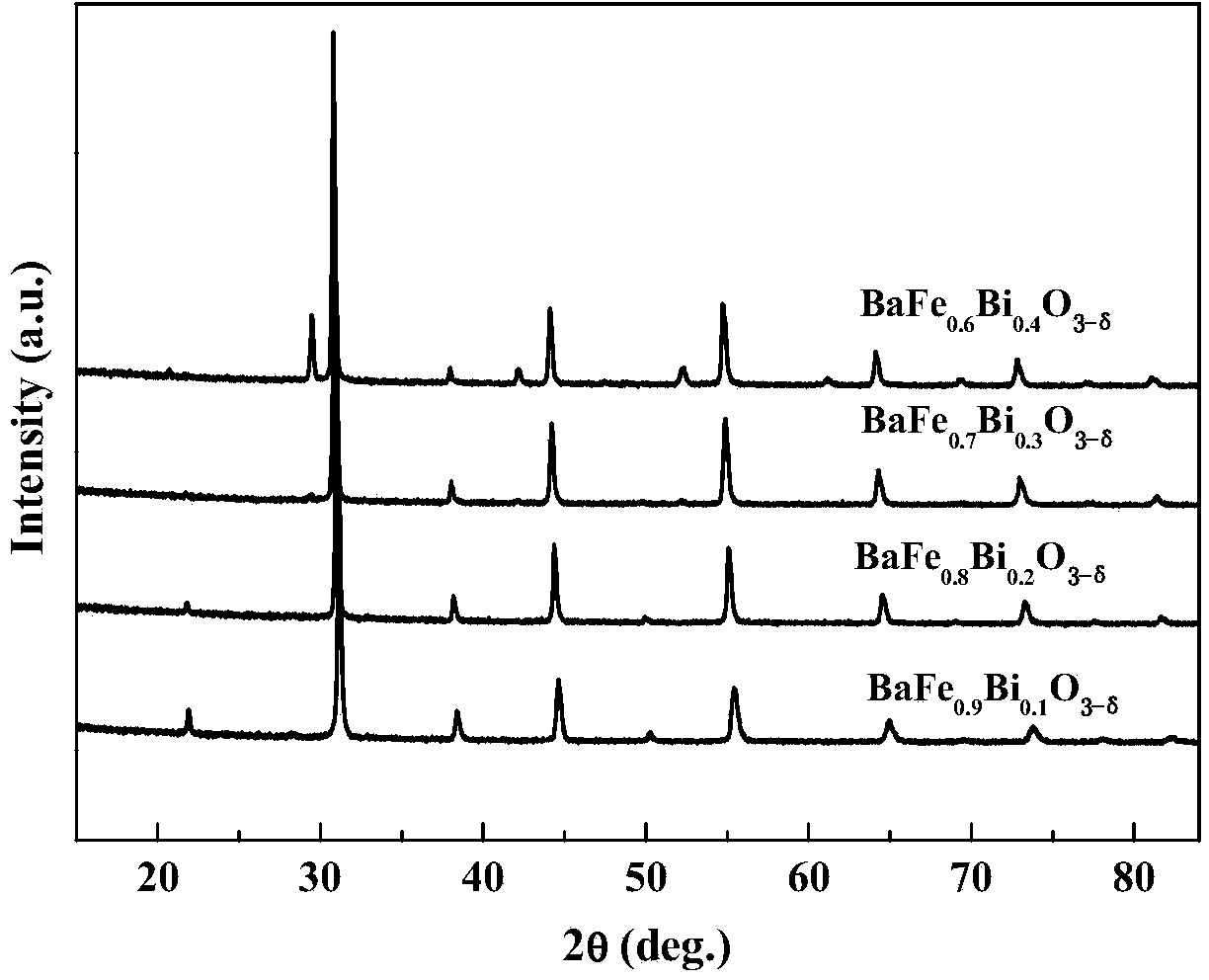
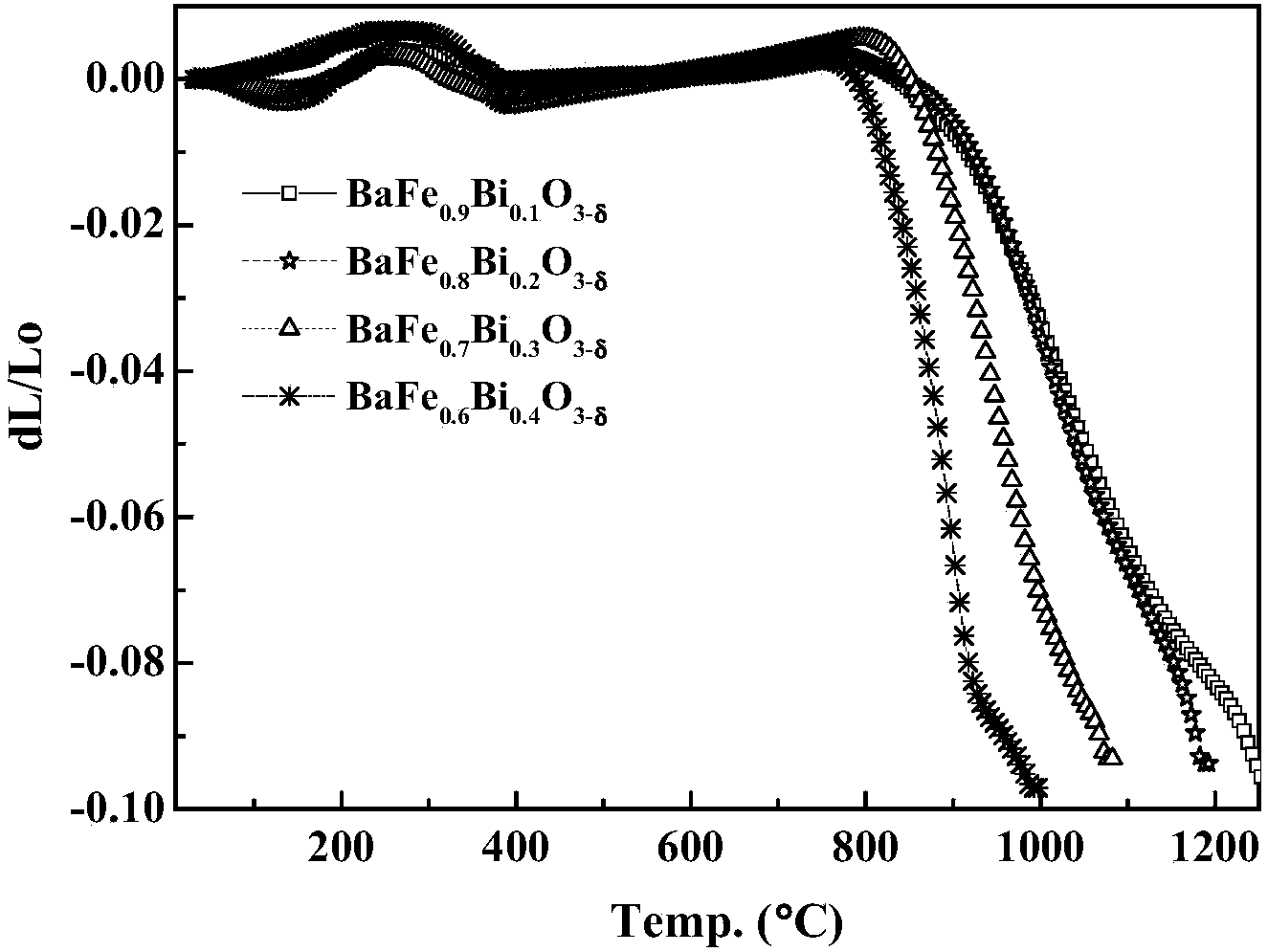
[0042] This embodiment provides a perovskite-type cathode material used in a medium-low temperature solid oxide fuel cell, whose chemical formula is BaFe 1-x Bi x o 3-δ , where δ represents oxygen excess or deficiency, -1≤δ≤1, x represents Bi 2 o 3 The amount of doping, 0≤x≤0.5.
[0043] The cathode material used by the solid oxide fuel cell represented by the above chemical formula is B-site Bi 2 o 3 Doped BaFO with perovskite crystal structure 3-δ , by Bi 2 o 3 The doping of doping improves the electrochemical performance, in order to prepare SOFC cathode materials with stable structure and suitable thermal expansion coefficient.
Embodiment 2
[0045] This embodiment provides a preparation and characterization method of the above-mentioned medium and low temperature solid oxide fuel cell cathode material, which specifically includes the following steps:
[0046] Step 1: according to the chemical formula of the material, weigh metal nitrate barium nitrate, iron nitrate and bismuth nitrate in stoichiometric formula ratio respectively as raw materials, and weigh a certain amount of ethylenediaminetetraacetic acid (EDTA) and citric acid as complex Mixture, the molar ratio of ethylenediaminetetraacetic acid: citric acid: metal ion is 1:1~2:1~2.
[0047] Step 2: dissolving ferric nitrate in a certain amount of distilled water to prepare a clear solution, dissolving EDTA in a certain amount of dilute ammonia solution to form a clear solution, dissolving the weighed bismuth nitrate with nitric acid solution to form a clear solution for later use.
[0048] Step 3: Add the ammonia solution of EDTA into the ferric nitrate solut...
Embodiment 3
[0053] This embodiment provides a BaFe 0.7 Bi 0.3 o 3-δ The scheme of preparation and performance characterization, specifically includes the following experimental steps:
[0054] Step 1: Take by weighing 10.454 grams of analytically pure barium nitrate, 11.312 grams of iron nitrate and 5.821 grams of bismuth nitrate, and simultaneously weigh the organic complexing agent according to the ratio of EDTA: citric acid: metal ion=1: 1.5: 1 23.379 grams of ethylenediamine tetraacetic acid, 25.217 grams of citric acid.
[0055] Step 2: Dissolve ferric nitrate with 100mL distilled water to form a reddish-brown solution; dissolve ethylenediaminetetraacetic acid with 30mL dilute ammonia water (the volume ratio of ammonia water and distilled water is 1:1) to form a transparent and clear EDTA-ammonia solution; Nitric acid (the volume ratio of nitric acid and distilled water is 2:1) dissolves bismuth nitrate to form a transparent and clear bismuth nitrate solution.
[0056] Step 3: Ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com