Anti-Listeria monocytogenes monoclonal antibody and its application
A technology of mononucleosis and monoclonal antibody, which is applied in the biological field, can solve the problems of increased detection cost, long detection cycle, and extended detection time, and achieve strict prevention of missed detection and false detection, good sensitivity, improved detection sensitivity and The effect of the detection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
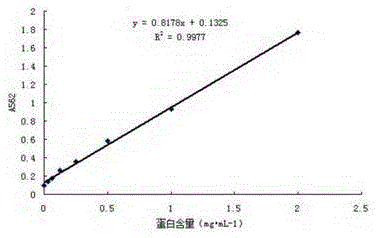
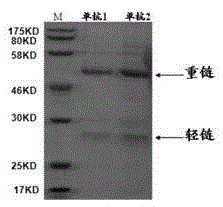
Image
Examples
Embodiment 1
[0019] Preparation of Example 1 Listeria monocytogenes Antigen Bacteria Liquid and BALB / c Mouse Immunization
[0020] Take the Listeria monocytogenes ( ATCC19111 ), inoculated on Trypticase Soy Yeast Extract Agar (TSA) medium (pH7.3±0.1) by streaking method, cultured in conventional method, centrifuged at 6000r / min for 10min, collected bacterial precipitate, and sterilized Colony counting (2.4×10 9 CFU / mL), adding formaldehyde with a final concentration of 0.3% overnight at 4°C to inactivate the bacteria. The next day, the eluted bacterial liquid was subjected to ultrasonic waves under the conditions of 20KHZ and 150W ice bath to break the bacterial cells, each time for 10 seconds, with an interval of 10 seconds, and a total time of 20 minutes. BCA protein assay kit was used to measure the bacterial protein content, and the result was 2.548 mg / mL.
[0021] Eight-week-old female BALB / c mice were used for immunization. The above-mentioned inactivated bacteria solution (2×...
Embodiment 2
[0022] The mensuration of embodiment 2 immune mouse serum antibody titer
[0023] After 3 immunizations, blood was collected from the tail of the mice, and the serum titer was detected by conventional indirect ELISA method. Wherein, the coated antigen is the Listeria monocytogenes bacterium liquid prepared in Example 1, the dilution is 1:40, the positive serum dilution is 1:1600, and the enzyme-labeled secondary antibody is HRP (horseradish peroxidase )-labeled rabbit anti-mouse IgG (1:15000 dilution). The assay results showed that the antibody titer was 1:12800.
Embodiment 3
[0024] Example 3 Preparation of Listeria monocytogenes monoclonal antibody
[0025] (1) Establishment of hybridoma cell lines
[0026] ①Preparation of feeder cells: Normal BABL / C mice were killed by neck dislocation, 8 mL of HAT culture solution was injected into the peritoneal cavity, and the culture solution was drawn out after gently shaking the mouse for a few times, and the cell concentration was adjusted to 10 5 Add 100 μL per well to a 96-well cell culture plate, place at 37°C, 5% CO 2 Cultivate overnight in a cell culture incubator for use as feeder cells.
[0027] ②Cultivation of myeloma cells and preparation of cell suspension: Myeloma cells (SP2 / 0) were resuscitated one week before fusion, passaged every other day, and fusion was carried out one day after passage. Take about 4 bottles (25cm 2 ) SP2 / 0 cells, blow down the cells in each bottle with RPMI-1640 culture medium, centrifuge at 1200r / min for 10min, repeat 2-3 times, and count with a cell counting board....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com