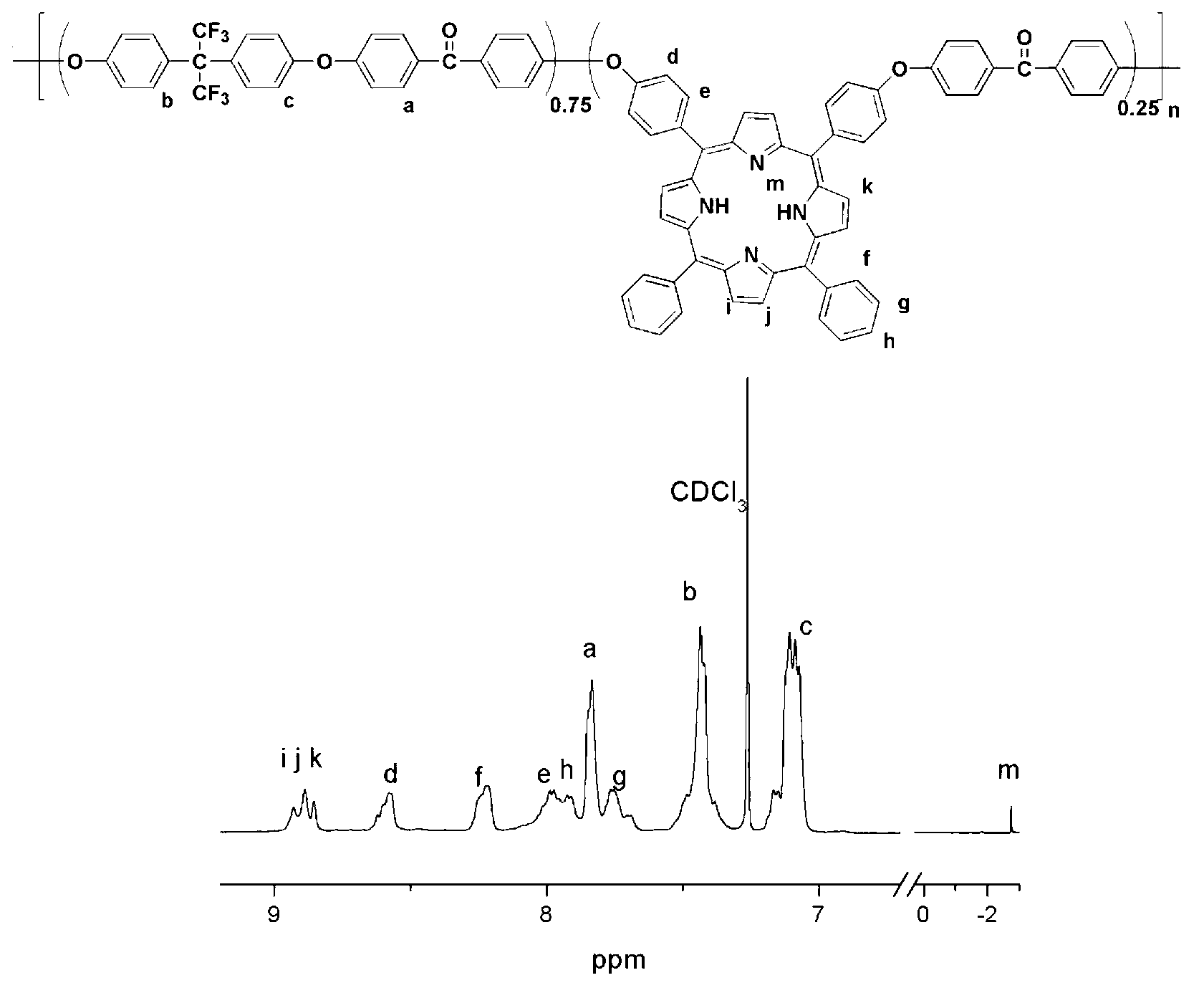
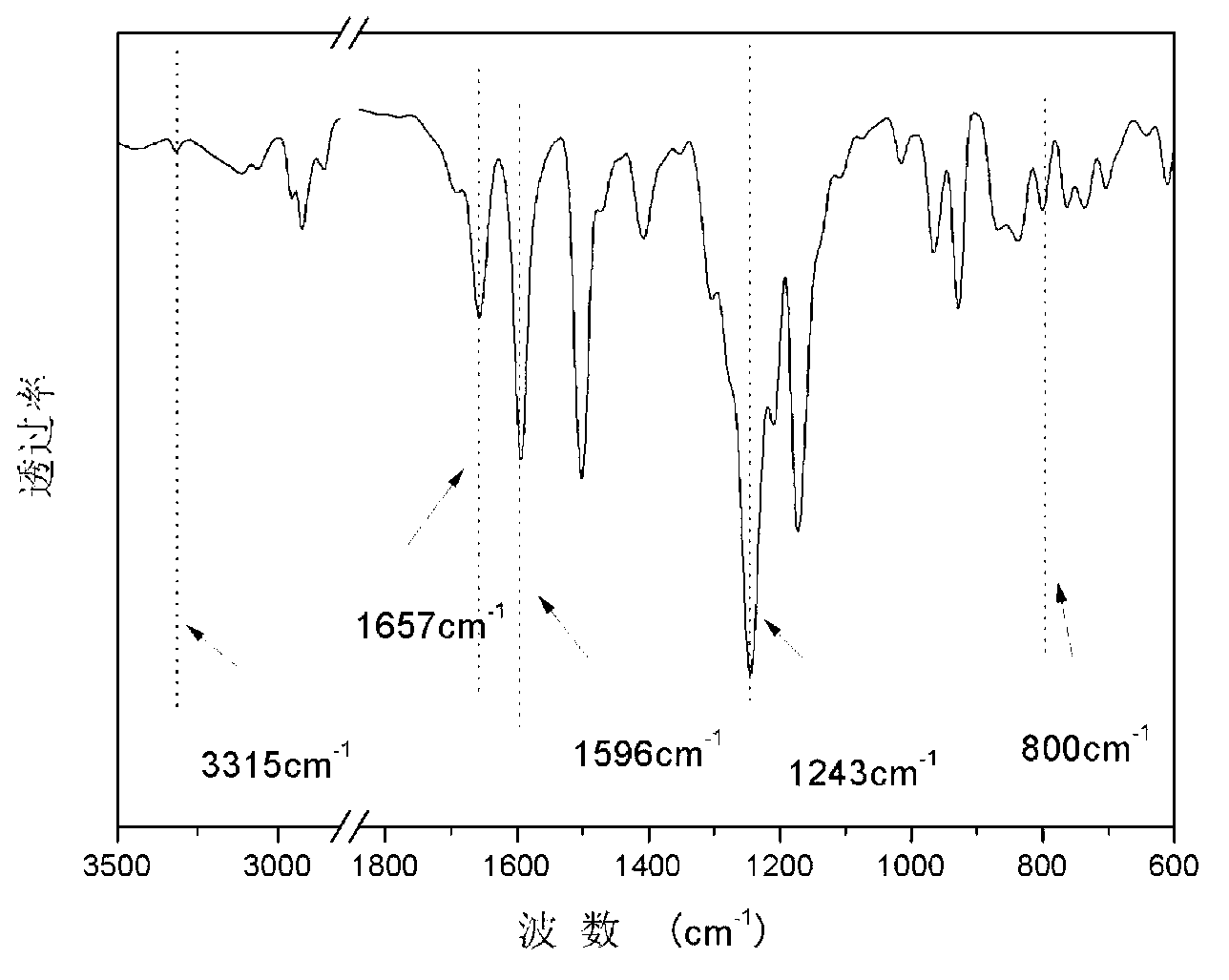
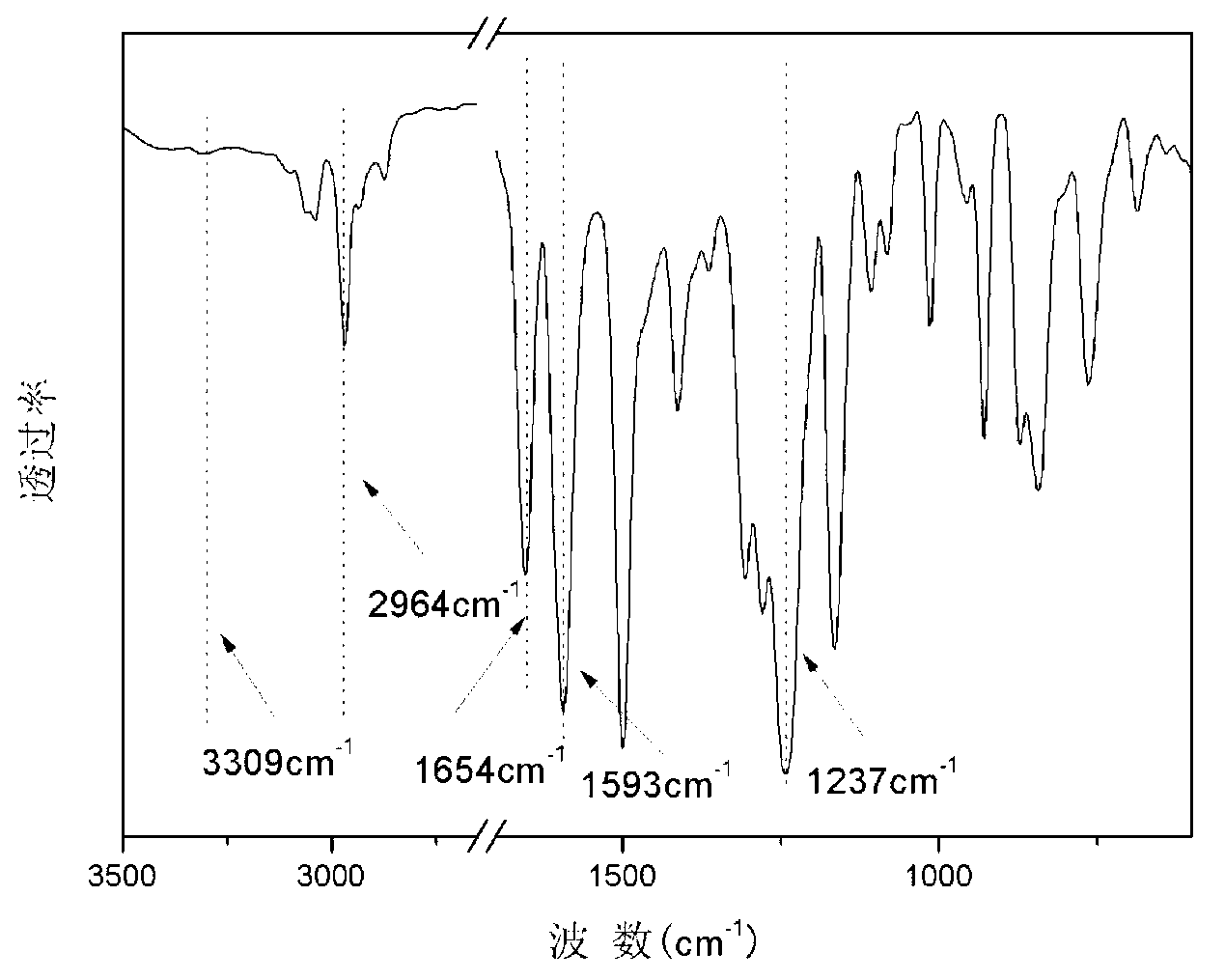
Polyaryletherketone copolymer containing porphyrin structure at main chain, and preparation method thereof
A technology of polyaryletherketone and copolymer, applied in the field of polyaryletherketone copolymer and its preparation, to achieve strong fluorescence response, avoid fluorescence quenching, and good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of 5,15-bis(4-hydroxyphenyl)-10,20-diphenylporphyrin and 5,10-bis(4-hydroxyphenyl)-15,20-diphenylporphyrin : Add 5.306g (50mmol) benzaldehyde and 6.106g (50mmol) 4-hydroxybenzaldehyde in a 1000ml three-necked flask, add 350ml propionic acid to dissolve, heat to reflux, add 6.94ml (100mmol) freshly steamed pyrrole drop by drop, 20 minutes After the dropwise addition is completed, continue to reflux for 40 minutes, add an equal volume of distilled water, and cool to room temperature. Neutralize the propionic acid in the system with saturated sodium carbonate solution, filter under reduced pressure, wash the filter cake with saturated sodium carbonate solution and distilled water several times, dissolve it with a mixed solvent of chloroform and ethanol, mix it into silica gel, spin dry and put it on the column. Wash the first and second color bands with chloroform as the eluting machine; then wash the third and fourth color bands with a solvent of 100:2 rat...
Embodiment 2
[0041] (1) The preparation of 5,10-bis(4-hydroxyphenyl)-15,20-diphenylporphyrin is the same as that described in Example 1.
[0042] (2) Add 5,10-bis(4-hydroxyphenyl)-15,20-diphenylporphyrin to a 50ml three-necked flask equipped with mechanical stirring, nitrogen vent, thermometer, water dispenser and condenser 0.323g (0.5mmol) of morphine, 2.169g (9.5mmol) of 2,2-di(4-hydroxyphenyl)propane, 2.182g (10mmol) of 4,4'-difluorobenzophenone and 1.656 anhydrous potassium carbonate g (12mmol) was dissolved in N-methylpyrrolidone (20ml), water-carrying agent toluene (11ml) was added, and nitrogen gas was passed through for 15 minutes until all the monomers were dissolved, then heated to 135°C and refluxed for 3 hours, and then the water-carrying agent was released. Agent toluene and the water that reaction generates, reaction system is warmed up to 170 ℃, finishes copolymerization reaction after 2.5 hours. The viscous solution was slowly poured into absolute ethanol to obtain flexibl...
Embodiment 3
[0045] (1) The preparation of 5,15-bis(4-hydroxyphenyl)-10,20-diphenylporphyrin was as described in Example 1.
[0046] (2) Add 5,15-bis(4-hydroxyphenyl)-10,20-diphenylporphyrin to a 50ml three-necked flask equipped with mechanical stirring, nitrogen vent, thermometer, water dispenser and condenser 0.972g (1.5mmol) of morphine, 2.858g (8.5mmol) of 2,2-bis(4-hydroxyphenyl)hexafluoropropane, 2.182g (10mmol) of 4,4′-difluorobenzophenone and anhydrous carbonic acid Potassium 1.656g (12mmol) was dissolved in N-methylpyrrolidone (24ml), water-carrying agent toluene (12ml) was added, nitrogen gas was passed through for 15 minutes and after the monomer was dissolved, the temperature was raised to 138°C and refluxed for 3 hours, and then the Aqueous toluene and the water generated by the reaction, the reaction system is heated up to 176 ° C, and the copolymerization reaction is completed after 2 hours. The viscous solution was slowly poured into absolute ethanol to obtain flexible pol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Young's modulus | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com