Diethyl phosphinate fire retardation agent preparation method
A technology of diethyl phosphinate and diethyl phosphinic acid is applied in the field of preparation of diethyl phosphinate flame retardants without monoethyl phosphinic acid group, and can solve the problem of no diethyl phosphinate. Problems such as detailed data of aluminum base phosphinate, complex process, and increased process procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
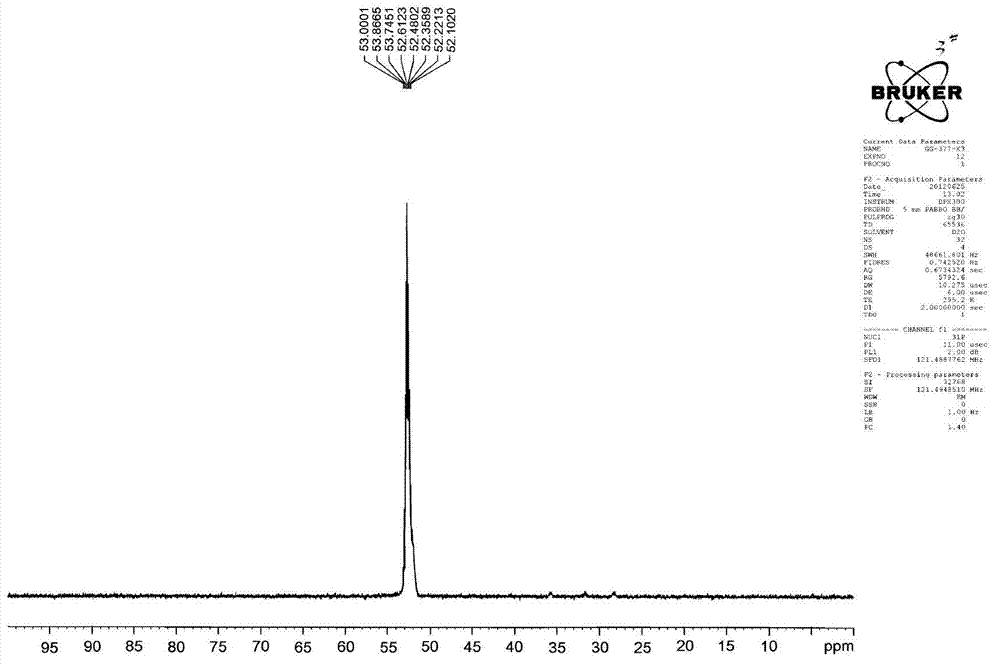
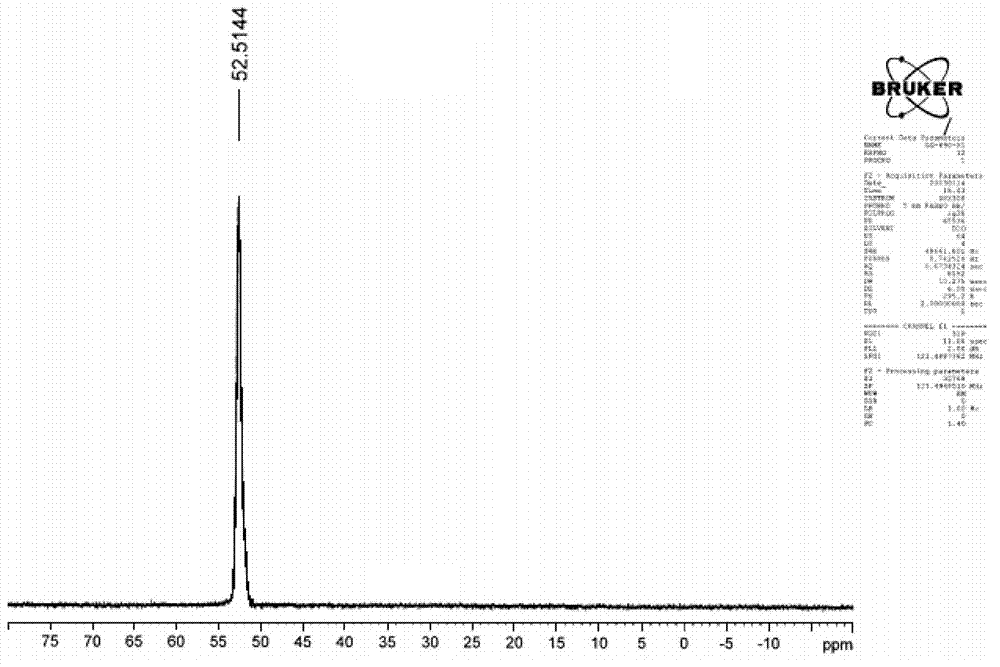
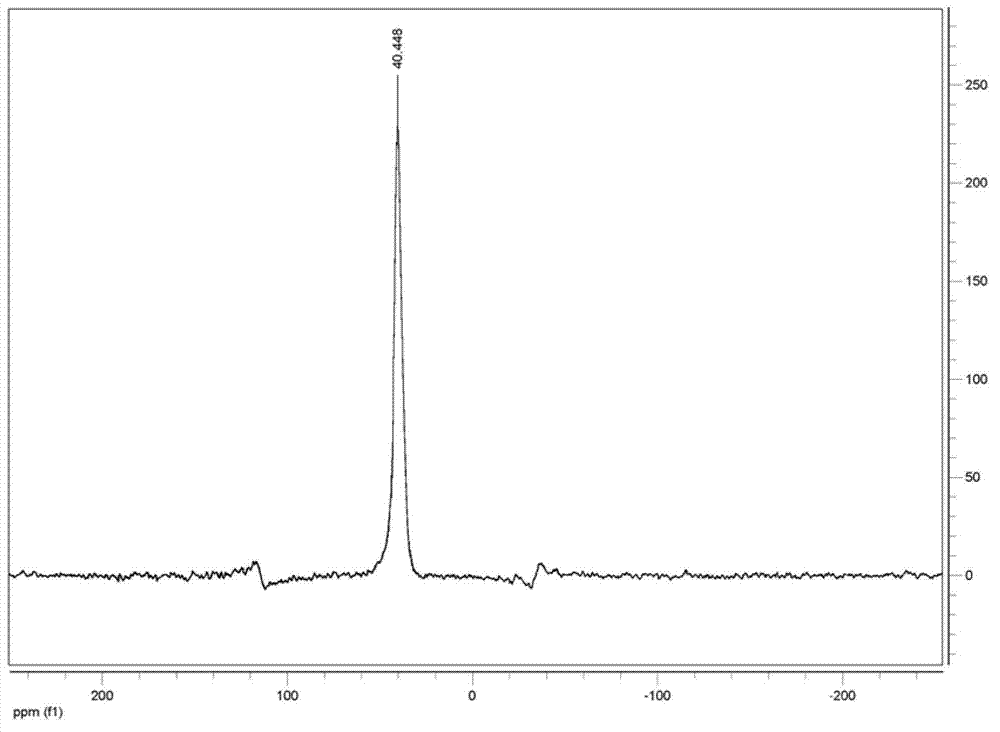
Image
Examples
Embodiment 1
[0048] Add 212g of sodium hypophosphite, 120g of acetic acid and 44g of ethylene glycol monomethyl ether into an 800mL acid-resistant and pressure-resistant reactor that can be heated and cooled, heat the materials in the reactor to 80°C, and pass ethylene into the reactor through a pressure reducing valve and make the pressure in the kettle reach 1MPa, add the mixture composed of 20g of azobisisobutyronitrile, 0.4g of copper complex and 44g of ethylene glycol monomethyl ether in batches under stirring at 800 rpm, add the initiator During the process, the ethylene pressure in the reactor should not be lower than 0.8MPa, the temperature should not be higher than 90°C, and the addition should be completed within 10 hours. Reactor is cooled to room temperature, excess ethylene is fed into bromine water to recover when the pressure is released, and finally the dissolved ethylene is driven out of the liquid with nitrogen to obtain a product with a total weight of 558g (theoretical 5...
Embodiment 2
[0054] Add 212g of sodium hypophosphite, 185g of propionic acid and 44g of ethylene glycol monobutyl ether into an 800mL acid-resistant and pressure-resistant reactor that can be heated and cooled, heat the materials in the reactor to 80°C, and feed ethylene into the reaction through a pressure reducing valve. In the kettle and make the pressure in the kettle reach 1MPa, add the mixture consisting of 20g of azobisisobutyronitrile, 0.4g of copper complex and 44g of ethylene glycol monobutyl ether in batches at 800 rpm, add the initiator During the process, the ethylene pressure in the reactor should not be lower than 0.6MPa, the temperature should not be higher than 95°C, and the addition should be completed within 10 hours. Reactor is cooled to room temperature, excess ethylene is fed into bromine water to reclaim during decompression, finally drives away dissolved ethylene in the liquid with nitrogen, obtains the product of total weight 589g (theoretical 582.4g), corresponding...
Embodiment 3
[0060] Add 424g of sodium hypophosphite, 250g of acetic acid and 100g of butyl ether into a 1500mL acid-resistant and pressure-resistant reactor that can be heated and cooled. When the internal pressure reaches 1.2MPa, add a mixture of 30g di-tert-butyl peroxide, 0.6g copper complex and 100g butyl ether in batches at 600 rpm, and keep the ethylene pressure in the reaction kettle during the process of adding the initiator. Lower than 0.6MPa, the temperature is not higher than 100°C, and the addition is completed within 16 hours. Reactor is cooled to room temperature, excess ethylene is fed into bromine water recovery during decompression, finally drives away the dissolved ethylene in the liquid with nitrogen, obtains the product of gross weight 1112.0g (theoretical 1108.6g), is equivalent to consuming 227.4g ethylene absorption ( Theoretical absorption of ethylene 224g).
[0061] The resulting product is moved into a still, and the mixture of butyl ether and acetic acid is rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com