Sorafenib nano particle and application thereof
A sorafenib nanometer and nanoparticle technology, applied in the direction of non-active ingredient medical preparations, powder delivery, antineoplastic drugs, etc., can solve intestinal, systemic or skin lesions, diarrhea, weight loss, hand- Foot skin reaction and other problems to achieve the effect of avoiding excessive drug release, good ball formation, and avoiding drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 mPEG-PDLLA
[0043] First, put polyethylene glycol monomethyl ether (Methoxypolyethylene glycols, mPEG for short) into the schlenk tube, and remove water under vacuum in the molten state; then add the previously refined racemic lactide (DLLA) and the catalyst stannous octoate The reaction tube was evacuated repeatedly, nitrogen was ventilated, and finally the tube was vacuum-sealed and placed in an oil bath at 140°C for 10 h. After the reaction, the product was dissolved with dichloromethane, then precipitated in cold ether, and the target product was obtained after vacuum drying. Among them, the polymer material (mPEG-PDLLA) is a polyethylene glycol monomethyl ether and racemic lactide copolymerized at a weight ratio of 1:1.1~2 (preferably 1.5:2.5). Methyl ether-polylactic acid block copolymer; its general formula is mPEG-PDLLA, its molecular weight range is 40-45kD, and its reaction route is as follows:
[0044]
[0045] Wherein, t...
Embodiment 2
[0046] Example 2 Preparation method of polyethylene glycol monomethyl ether-polylactic acid block copolymer Sorafenib nanoparticles
[0047] The raw material formula is as follows:
[0048] Tetrahydrofuran (C 4 h 8 O) 12ml
[0049] Polymer material (mPEG-PDLLA) 0.25g
[0050] Sorafenib 12.5mg
[0051] Methanol (CH 3 OH) 6ml
[0052] Make 18ml Sorafenib Nanoemulsion
[0053] Stir the above mixture to form a clear and transparent solution, then vacuum rotary evaporate at 60°C, remove the organic solvent, add 50 mL of distilled water at 60°C, rotate at normal pressure for 10 minutes, centrifuge for 10 minutes (4000 r / min), Get the supernatant and be the required Sorafenib nanoparticle solution, 60 Co irradiated and sterilized, and stored in a 4°C refrigerator for later use.
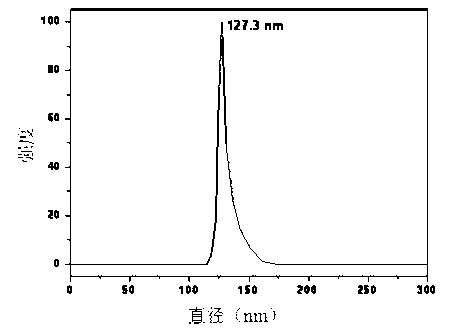
[0054] The detection results of Sorafenib nanoparticles show that the particle size is uniform and the shape is round, such as Figure 1a , Figure 1b As shown, the particle size is 127.3±2.0 nm,...
Embodiment 3
[0055] Example 3 In vitro drug release test of polyethylene glycol monomethyl ether-polylactic acid block copolymer sorafenib nanoparticles
[0056] Prepare a solution of phosphate buffered saline (PBS) (pH7.4, 0.01M) of sorafenib drug-loaded nanoparticles with a concentration of 0.5 wt%, then measure 5 ml and put it into a dialysis bag (molecular weight cut-off 3500). Put the dialysis bag into a 50 ml volumetric flask, add 35 ml of PBS, seal it and place it in a constant temperature air bath shaker (37°C, 75 rpm). At 2, 4, 8, 16, 24, 48, 72, 96, 120, 144, and 168 hours after the release, 20 ml of the release solution was sampled, and an equal amount of fresh PBS solution was added. The UV absorption value of the sample was measured at a wavelength of 263nm, and the concentration and cumulative release percentage of Sorafenib in the release medium were calculated.
[0057] In addition, a sorafenib PBS solution with a concentration of 0.5 wt% was prepared, 5.0ml of the solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com