A kind of preparation method of strychnine immune nanoparticles
A strychnine and nanoparticle technology, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of large dosage and strong toxic and side effects, and achieve drug release. Stable, good biocompatibility, good spheroidizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation method of anti-human AFP McAb-polyethylene glycol-polylactic acid block copolymer strychnine immune nanoparticles
[0048] The raw material formula is as follows:
[0049]
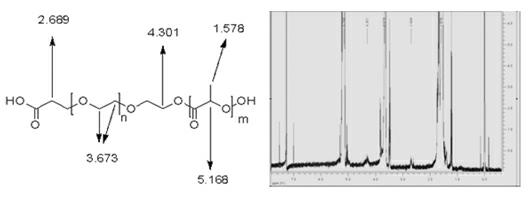
[0050] Among them, the polymer material (PLA-PEG-CH2-CH2-COOH) is a carboxylated polyethylene glycol formed by copolymerization of carboxylated polyethylene glycol and polylactic acid at a weight ratio of 1:1.5~3 (preferably 1.5:2.5). Diol-polylactic acid block copolymer; its general formula is PLA-PEG-CH2-CH2-COOH, its molecular weight range is 40-50kD, its structural fragments and 1 H-NMR measurement spectrum such as figure 1 shown.
[0051] Mix the above-mentioned oil phase and water phase, disperse with a high-shear homogenizer, shear at 12000 rpm for 5 minutes to obtain a dispersion, and then continue to emulsify the dispersion under 300w power ultrasonic conditions, ultrasonic 5 times, each 30s, Get first emulsion. Pour the primary emulsion into the diluent of the prescripti...
Embodiment 2
[0055] In vitro drug release test of anti-human AFP McAb-polyethylene glycol-polylactic acid block copolymer strychnine immune nanoparticles
[0056] Precisely pipette 2.0ml of strychnine immune nanoparticle concentrate, place it in a dialysis bag with a molecular weight cut-off of 3,000 Daltons, tie both ends tightly, operate 3 parts in parallel, and put it into 50ml of PBS medium with pH7.4 In vitro release at 37°C and 100rpm rotation speed, 4ml samples were taken at 0.5, 1, 2, 4, 8, 12, 16, 18, 24, 36, 48h after the start of the release, 4ml of rehydration solution was taken, and the samples were released at a wavelength of 263nm Determine the UV absorption value, calculate the concentration of Bru (strichnine) in the release medium and the cumulative release percentage.
[0057] Another Bru-PBS solution with a concentration of 0.427 mg / ml was prepared, 2.0 ml of the solution was precisely pipetted, placed in a dialysis bag with a molecular weight cut-off of 3,000 Daltons, ...
Embodiment 3
[0060] Determination of targeting of anti-human AFP McAb-polyethylene glycol-polylactic acid block copolymer strychnine immune nanoparticles
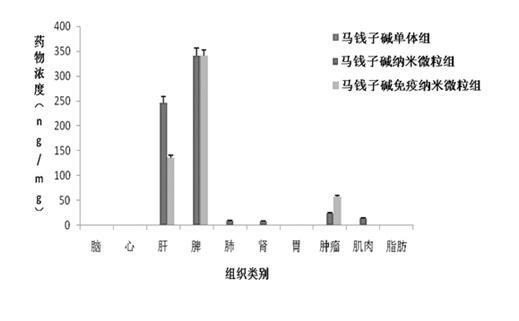
[0061] Anti-human AFP McAb strychnine immune nanoparticles were incubated with liver cancer cells SMMC-7721 for 4 h, and the cells were washed 3 times with 0.01M PBS; FITC-labeled secondary antibody was added, incubated for 2 h, and washed 3 times with PBS; Complementary immunofluorescence methods were observed under a confocal microscope. A 200X confocal microscope showed that strychnine immune nanoparticles were evenly distributed near the liver cancer cell membrane, showing an approximate "ring" shape, showing good targeting, as shown in Figure 5 Shown (photograph under confocal microscope × 200 times).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com