Plant anti-cancer targeting nano-preparation, and preparation method thereof
A nano-preparation, plant-based technology, applied in the field of medicine, can solve the problems of slow dissociation of complexes, unstable carriers, limited tumor efficacy, etc., and achieve good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 prepares paclitaxel / TPGS / hyaluronic acid / albumin nano-preparation
[0048] (1) Dissolve 500mg of human serum albumin freeze-dried powder and 500mg of hyaluronic acid with a molecular weight of 5000Da in 20mL of PBS buffer; adjust the pH of the solution to 5.5 to obtain an albumin / hyaluronic acid solution;
[0049] (2) Dissolve 50 mg of paclitaxel and 50 mg of TPGS in 1 mL of chloroform; add the paclitaxel solution to the above albumin / hyaluronic acid solution under stirring conditions to form colostrum;
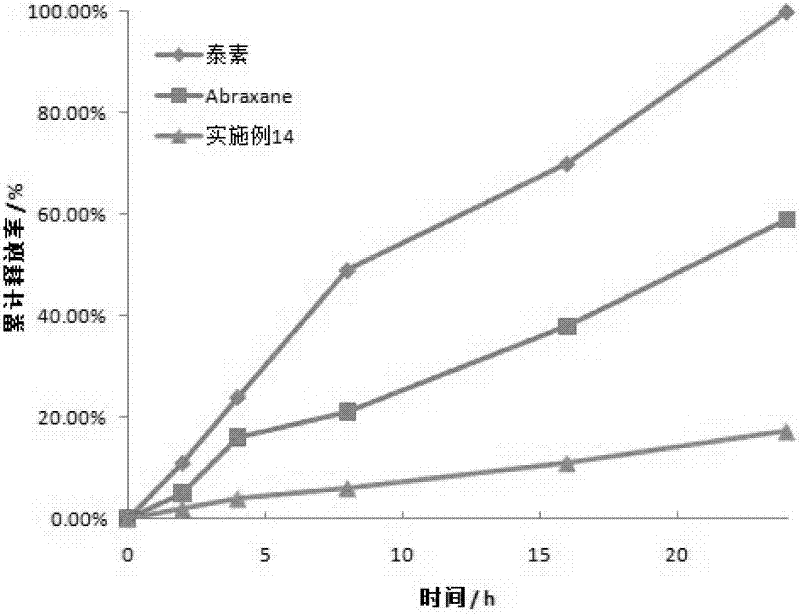
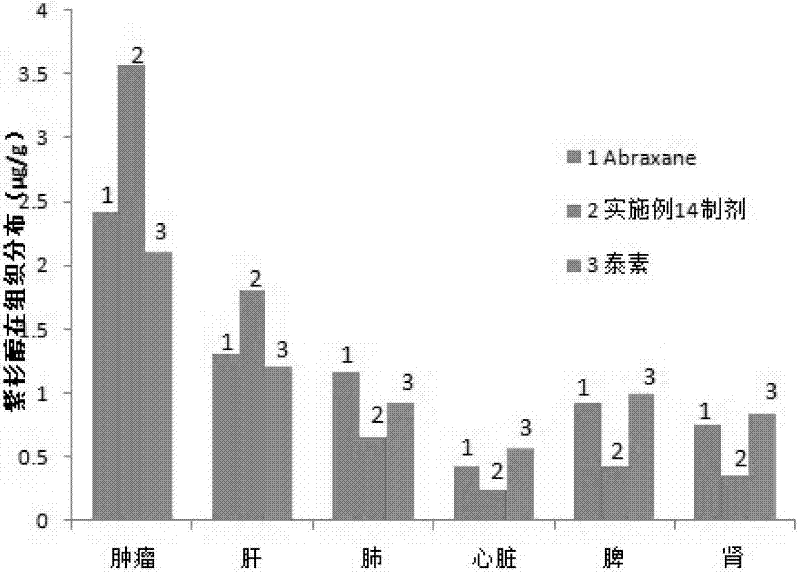
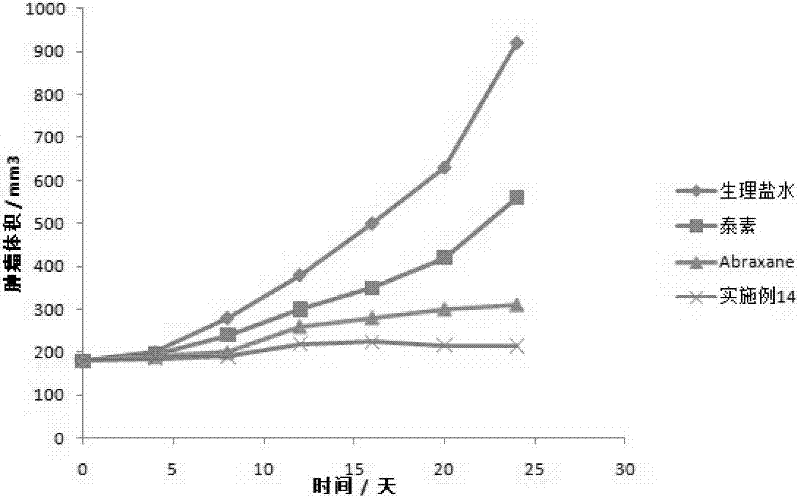
[0050] (3) The colostrum is processed by a high-pressure homogenizer (9000-40000psi) to obtain a nanoemulsion. The nanoemulsion is transferred to a rotary evaporator and evaporated under reduced pressure at 30-45°C to quickly remove the organic solvent, and freeze-dried under sterile conditions to obtain a freeze-dried product. Powder; lyophilized powder is stable at room temperature for less than 10 hours after resuspended in saline. Measured by a nanome...
Embodiment 2
[0051] Embodiment 2 prepares paclitaxel / TPGS / hyaluronic acid-albumin nano-preparation
[0052] (1) Weigh 500 mg of human serum albumin freeze-dried powder and dissolve it in 20 mL of sterile water; add 400 mg of hyaluronic acid with a molecular weight of 5 KDa to the albumin solution, and dissolve the albumin completely under full stirring; then, use 0.1 N hydrochloric acid to adjust the pH of the solution to 5.5; EDCI, which is twice the number of moles of hyaluronic acid, was added to the above solution, and stirred overnight at room temperature; The reaction product of EDCI and EDCI was then freeze-dried; the above freeze-dried hyaluronic acid-albumin conjugate was dissolved in 20 mL of PBS buffer; the pH of the solution was adjusted to 5.5 to obtain an albumin / hyaluronic acid solution;
[0053] (2) Dissolve 50 mg of paclitaxel and 50 mg of TPGS in 1 mL of chloroform; add the paclitaxel solution to the albumin / hyaluronic acid solution under stirring conditions to form colos...
Embodiment 3
[0055] Example 3 Preparation of paclitaxel / TPGS / hyaluronic acid-albumin nano-preparation
[0056] (1) Preparation of hyaluronic acid-albumin conjugate
[0057] Prepare hyaluronic acid active lipid first: dissolve 400 mg of hyaluronic acid (molecular weight 5KDa) in 10 mL of 0.1M MES buffer, add 1.2 times the molar number of hyaluronic acid EDCI, and 1.2 times the molar number of hyaluronic acid N -Hydroxysulfosuccinimide; stir and react at room temperature for 30 minutes to prepare hyaluronic acid succinimide active lipid;
[0058] Dissolve 500 mg of human serum albumin freeze-dried powder in 10 mL of sterile water, add it to the succinimide active lipid solution of hyaluronic acid; adjust the pH value to 7.0-7.2, and stir and react at room temperature for 60 minutes; after the reaction is completed Add it into a dialysis membrane with a molecular weight of 10,000, dialyze to remove unreacted reagents and reaction by-products; freeze-dry the hyaluronic acid-albumin solution a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com