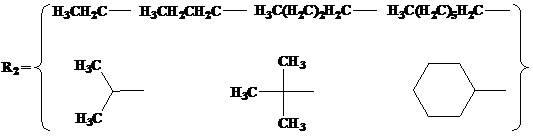
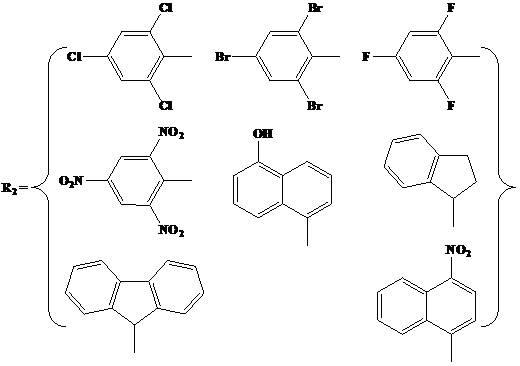
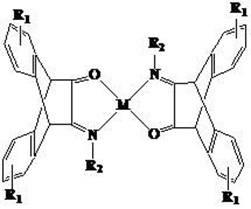
N,O-double ligand metal catalyst with stereo structure and preparation method
A metal catalyst and three-dimensional structure technology, applied in the field of the main catalyst of the catalytic system, can solve the problems of poor resistance to polar groups, reduced activity, etc., and achieve the effects of high light transmittance, low moisture absorption rate, and good processing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (I) Preparation of α-ketophenylimine ligands with novel stereostructures.
[0039] Add 0.0143 mol of 9,10-dihydro-9,10-ethylene anthracene-11,12-dione, 0.0143 mol of aniline, and a catalytic amount of p-toluene into the reaction flask equipped with a reflux condenser and a water separator Sulfonic acid and 150 mL of toluene were used as solvents. After 2 hours of reaction, recrystallize from n-hexane or distill under reduced pressure to obtain a novel stereoscopic α-ketobenimine ligand with a purity of more than 99%.
[0040] (II) Preparation of bis(α-ketophenylimine)nickel(II) with novel stereostructure as main catalyst.
[0041] 50 o Add 0.014 mol of the ligand prepared in step I to the tert-butanol solution containing 0.01 mol of potassium tert-butoxide at C. After the reaction solution is clear and transparent, lower the temperature of the system to room temperature, and then add 0.007 mol of tetraethylamine tetra Nickel bromide was kept at room temperature for 3...
Embodiment 2
[0045] (I) Preparation of α-ketophenylimine ligands with novel stereostructure:
[0046] With embodiment 1.
[0047] (II) Preparation of bis(α-ketophenylimine)palladium(II) with novel stereostructure as main catalyst.
[0048] 50 o Add 0.014 mol of the ligand prepared in step I to the tert-butanol solution containing 0.01 mol of potassium tert-butoxide at C. After the reaction solution is clear and transparent, lower the temperature of the system to room temperature, and then add 0.007 mol of tetraethylamine tetra Palladium chlorate, kept at room temperature for 3 hours, filtered, and recrystallized to obtain bis(α-ketophenylimine)palladium(II) complex with a new stereostructure.
[0049] (III) Catalytic polymerization of norbornene.
[0050] Heat the reaction bottle equipped with a magnetic stirrer and a branch with a heat gun, and simultaneously vacuumize it, fill it with nitrogen, and replace it three times to ensure that the reaction system is in a dry nitrogen environ...
Embodiment 3
[0052] (I) Preparation of α-keto(2,6-dimethyl)phenylimine ligand with novel stereostructure.
[0053] Add 0.0143 mol of 9,10-dihydro-9,10-ethylene anthracene-11,12-dione, 0.0143 mol of 2,6-dimethyl Aniline, a catalytic amount of p-toluenesulfonic acid and 200 mL of toluene were used as solvents. After reacting for 4 hours, the α-keto(2,6-dimethyl)imine ligand with a new stereostructure with a purity of more than 99% was obtained by recrystallization from n-hexane or distillation under reduced pressure.
[0054] (II) Preparation of bis[α-keto(2,6-dimethyl)phenylimine]nickel(II) complexes with novel stereostructures as main catalysts.
[0055] 50 o Add 0.014 mol of the ligand prepared in step I to a tert-butanol solution containing 0.01 mol of potassium tert-butoxide at C. After the reaction solution is clear and transparent, lower the temperature of the system to room temperature, and then add 0.007 mol of tetraethylamine tetra Nickel bromide was kept at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com