Method for removing NO and SO2 from environment pollution emissions
A technology of SO2 and pollutants, applied in the field of electrochemistry, can solve the problems of large investment, high operating costs, and increased difficulty, and achieve the effects of reduced transformation costs, saving operating costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
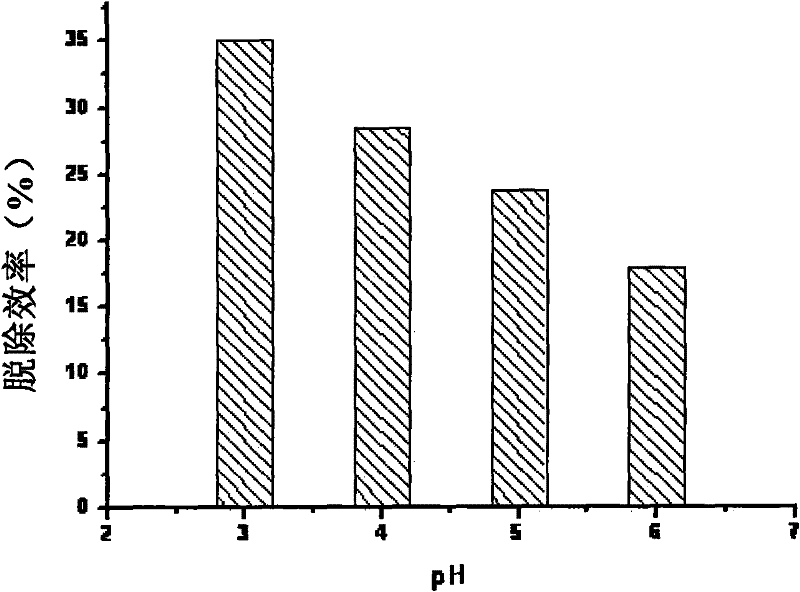
[0058] The test of the influence of embodiment 1 initial pH on denitrification efficiency
[0059] Experimental Materials:
[0060] Fe powder: 100 mesh, the required purity is 90%; carbon powder: 200 mesh, the required purity is 90%; N 2 For commercially available gas: the content is 99.9%; NO is commercially available gas: the content is 99.9%; O 2 It is a commercially available gas: the content is 99.9%; double distilled water: 400 mL; HCl is a commercially available pure reagent for chemical analysis.
[0061] Steps:
[0062] (1) Accurately weigh 28 grams each of iron powder and carbon powder, add the mixture of iron powder and carbon powder into a container filled with 400 mL of water, add HCl to adjust the initial pH, and mix well;
[0063] (2) use N 2 Gas as the balance gas will NO, O 2 The mixed gas of gas passes through the gas valve and flow meter to control the flow rate and then passes into the above mixed liquid;
[0064] (3) After reacting for 30 minutes, NO...
Embodiment 2
[0069] Example 2 Adding complexing agent EDTA to the test of the influence of NO removal effect
[0070] Experimental Materials:
[0071] Fe powder: 100 mesh, the required purity is 90%; carbon powder: 200 mesh, the required purity is 90%; N 2 For commercially available gas: the content is 99.9%; NO is commercially available gas: the content is 99.9%; O 2 It is a commercially available gas: the content is 99.9%; double distilled water: 400 mL; HCl is a commercially available pure reagent for chemical analysis.
[0072] Experimental steps:
[0073] (1) Accurately weigh 56 grams of iron powder and carbon powder, add the mixture of iron powder and carbon powder into a container filled with 400 mL of water, add HCl to adjust the initial pH=3.0, and mix well;
[0074] (2) Use N2 gas as the balance gas to remove NO, O 2 The air-gas mixture passes through the air valve and the flow meter to control the flow rate and automatically flows into the above-mentioned mixed liquid;
[0...
Embodiment 3
[0081] Embodiment 3 removes SO alone 2 the test
[0082] Experimental Materials:
[0083] Fe powder: 200 mesh, the required purity is 99%; carbon powder: 200 mesh, the required purity is 90%; SO 2 For commercially available gas: the content is 99.9%; NO is commercially available gas: the content is 99.9%; O 2 Commercially available gas: the content is 99.9%; double distilled water: 400 mL; oxalic acid is a commercially available pure reagent for chemical analysis.
[0084] Experimental steps:
[0085] (1) Accurately weigh 56 grams each of iron powder and carbon powder, add the mixture of iron powder and carbon powder into a container filled with 400 mL of water, add oxalic acid to adjust the initial pH to 3.0, and mix well;
[0086] (2) use N 2 Gas as the balance gas will NO, O 2 The air-gas mixture passes through the air valve and the flow meter to control the flow rate and automatically flows into the above-mentioned mixed liquid;
[0087] (3) After reacting for 30 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com