Colchicine double-layer osmotic pump controlled release tablets and preparation method thereof
An osmotic pump controlled-release, colchicine technology, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of multiple administrations, and achieve the effect of reducing the number of administrations, stabilizing the drug release rate, and ensuring the therapeutic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149]
[0150] Coating film formula: cellulose acetate (CA-398-3) 16g
[0151] Polyethylene glycol (PEG-6000) 4g
[0152] Acetone 850mL
[0153] Ethanol (absolute ethanol) 150mL
[0154] The weight gain of the coating film is: 15 mg. The diameter of the drug release hole: 0.8 mm.
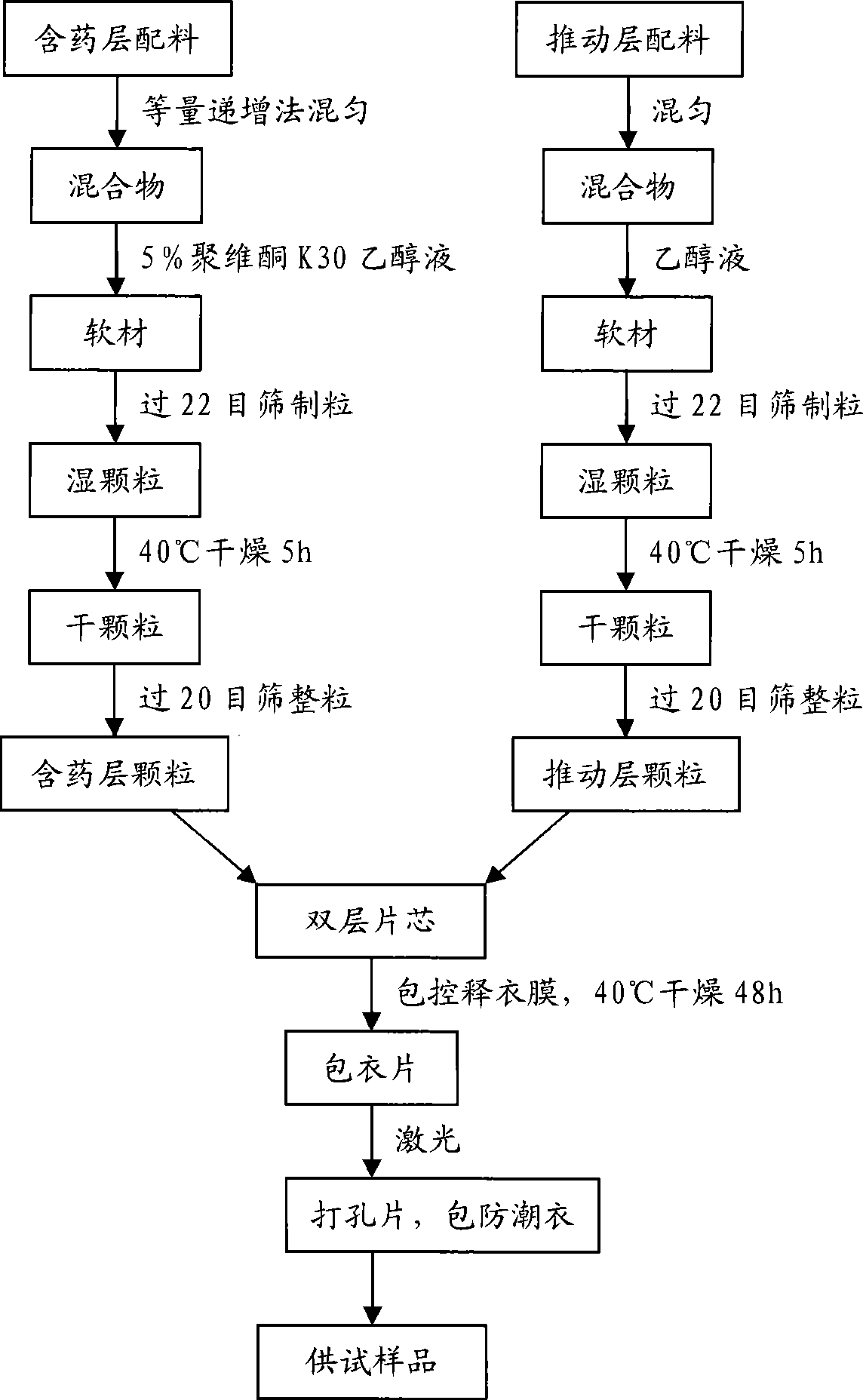
[0155] Preparation:
[0156] a. Mix colchicine, suspending agent, osmotically active substance and other pharmaceutical excipients according to the method of equal increment to make a mixture;
[0157] b. the mixture is mixed with PVPK-30 ethanol solution to make a soft material, the amount of the added ethanol solution is kneaded into agglomerates with the obtained soft material, and it is scattered as soon as it is waved; then granulated with a 22 mesh sieve to obtain a wet Granules, dry the wet granules at 40°C for 5 hours to obtain dry granules; then use a 20-mesh sieve to sieve the granules to obtain drug-containing layer granules;
[0158] c. Add expa...
Embodiment 2
[0163]
[0164]
[0165] Coating film formula: hydroxypropyl methylcellulose 19.4g
[0166] Polyethylene glycol (PEG-6000) 0.6g
[0167] Acetone 850mL
[0168] Ethanol (absolute ethanol) 150mL
[0169] The weight gain of the coating film is: 18mg. The pore diameter of the drug release hole: 2mm.
[0170] Preparation method: replace the film-forming material in the coating film with hydroxypropyl methylcellulose, and the rest of the preparation method is the same as in Example 1.
Embodiment 3
[0172]
[0173] Coating film formula: hydroxypropyl methylcellulose 19.4g
[0174] Polyethylene glycol (PEG-6000) 0.6g
[0175] Acetone 850mL
[0176] Ethanol (absolute ethanol) 150mL
[0177] The weight gain of the coating film is: 18 mg. The diameter of the drug release hole: 1.2 mm.
[0178] Preparation method: replace the film-forming material in the coating film with hydroxypropyl methylcellulose, and the rest of the preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore diameter | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com