Paclitaxel freeze drying microemulsion for injection and method of producing the same
A technology of paclitaxel and microemulsion, applied in the field of medicine, can solve the problems of restricting the wide use of paclitaxel inclusion compounds, not easy to degrade, embolism, etc., achieves improvement of bioavailability and therapeutic index, simple and easy preparation process, and avoids short storage period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
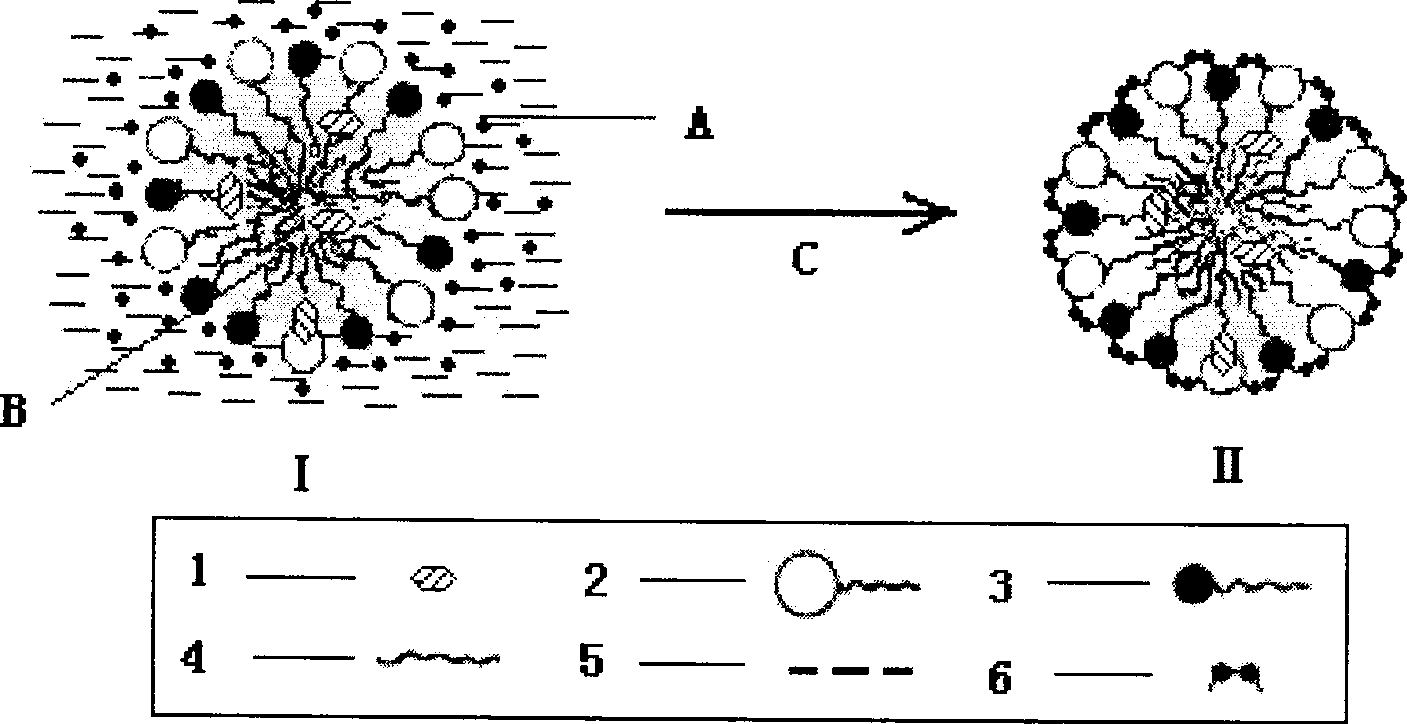
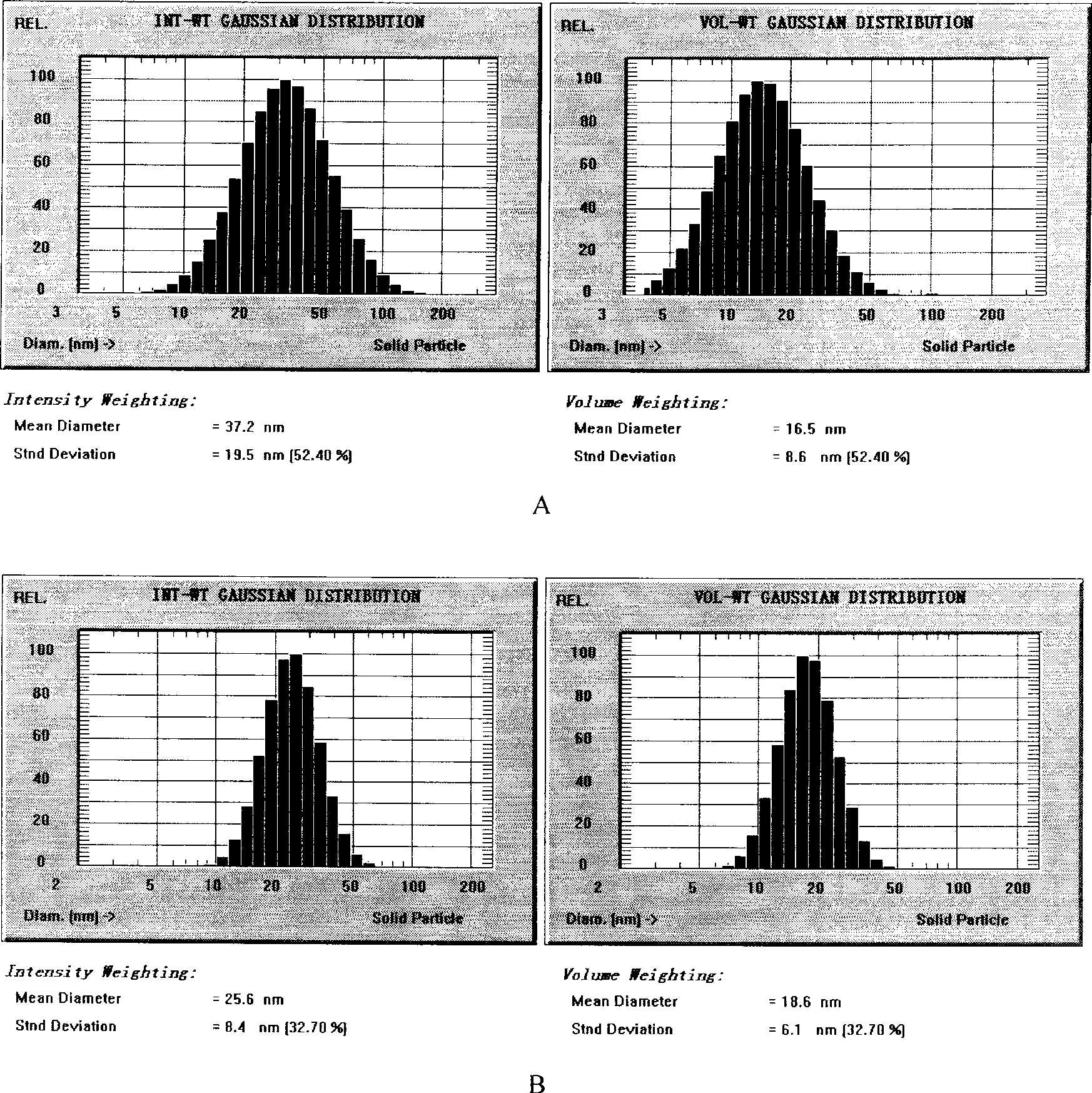
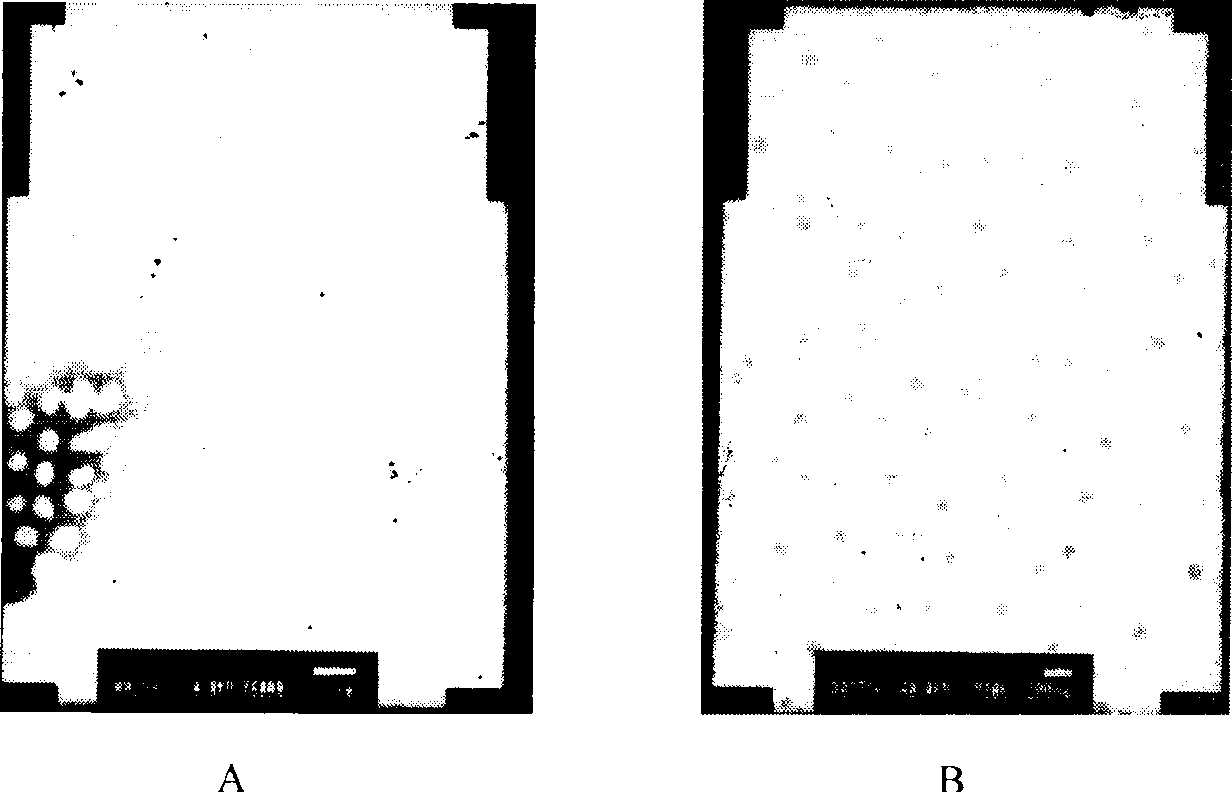
[0038] (1) Weigh 50g of soybean oil for injection, 12g of soybean lecithin, 2.0g of vitamin E, and 10g of paclitaxel, heat and stir in a water bath to melt, and keep the temperature at 70°C as the oil phase; (2) Weigh 1000 g of polyethylene glycol Add 15g of E succinate (TPGS), 1884.0g of Poloxamer, and 9.5g of sorbitol into an appropriate amount of water for injection, heat and stir to dissolve, and keep the temperature at 70°C as the water phase. Drop the water phase into the oil phase under stirring, continue to stir to form a microemulsion or form a nanoemulsion through high-pressure 750bar high-pressure emulsification 6 times, and adjust the pH to 5.5 with 0.2% citric acid; (3) in the above paclitaxel microemulsion Add 15% sucrose, pre-freeze at -80°C for 8 hours, and vacuum freeze-dry for 72 hours to obtain paclitaxel freeze-dried microemulsion for injection. After the lyophilized preparation was reconstituted with water, the particle size was 37.2nm, and the encapsulati...
Embodiment 2
[0041] (1) Weigh 35g of medium-chain oil, 20g of egg yolk phospholipid, 18g of paclitaxel, heat and stir in a water bath to melt, and keep the temperature at 65°C as the oil phase; (2) Weigh 25g of ethylene glycol monoethyl ether (Transcutol), Add 3.0 g of sodium deoxycholate, 8.5 g of mannitol, and 1.0 g of ascorbic acid into an appropriate amount of water for injection, heat and stir to dissolve, and keep the temperature at 65°C as the water phase. Drop the water phase into the oil phase under stirring, continue to stir to form a microemulsion, adjust the pH to 7.0 with 0.1mol / L NaOH; (3) add 20% trehalose to the above-mentioned paclitaxel microemulsion, and pre- After freezing for 7 hours, vacuum freeze-drying for 64 hours to obtain paclitaxel freeze-dried microemulsion for injection. After the lyophilized preparation was reconstituted with water, the encapsulation rate was 90.1%, and the particle size was 35.5nm. There was no precipitation within 48 hours after dilution wi...
Embodiment 3
[0044] (1) Weigh 45 g of ethyl oleate, 25 g of soybean oil, 15 g of soybean lecithin, and 6.0 g of paclitaxel in a water bath, heat and stir to melt, and keep the temperature at 75°C as the oil phase; (2) weigh polyethylene glycol-diglycol Add 2.5g of stearyl ethanolamine (PEG-DSPE), 1885.0g of Poloxamer, 1.2g of sodium sulfite, and 10.0g of glycerin into an appropriate amount of water for injection, heat and stir to dissolve, and keep the temperature at 75°C as the water phase. Drop the water phase into the oil phase under stirring, continue stirring to form a microemulsion, adjust the pH to 5.5 with 0.5mol / L phosphoric acid; (3) add 12% mannitol to the above paclitaxel microemulsion, and pre- Freeze for 12 hours, then vacuum freeze-dry for 80 hours to obtain paclitaxel freeze-dried microemulsion for injection. After the lyophilized preparation was reconstituted with water, the encapsulation rate was 89.3%, and the particle size was 30.1nm. There was no precipitation within 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com