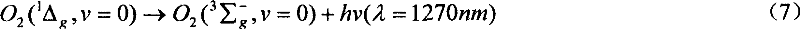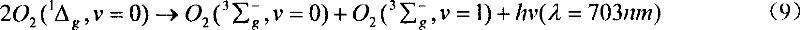Method for creating singlet oxygen by using solid peroxide or hyperoxide as chemical source
A technology of solid peroxide and superoxide, applied in the direction of oxygen preparation, etc., can solve the problems of slow reaction rate, low output rate, low singlet oxygen production efficiency, etc., and achieve the effect of easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: sodium peroxide-chlorine system: Na 2 o 2 -Cl 2
[0055] In a self-processing stainless steel fixed bed reactor, first spread 4-6mm glass beads on the bottom of the reactor, just to bury the inlet distribution pipe, and add 40g of sodium peroxide powder on the glass beads , and then add a small amount of glass beads on the powder layer to prevent the air flow from blowing away the sodium peroxide powder layer, vacuumize the reactor to keep the reactor pressure at about 40torr, and then react with chlorine gas. Directly align the optical fiber head coupled with OMA-V spectrometer and CCD spectrometer to the test window in the reactor to monitor the radiation signal of singlet oxygen in the gas phase. The spectrometer can easily monitor the near-infrared 1270nm of singlet oxygen Single-molecule radiation signals, but no bimolecular synergistic radiation signals at 6340nm or 703nm in the visible region.
Embodiment 2
[0056] Embodiment 2: lithium peroxide-carbon tetrachloride-chlorine system: Li 2 o 2 -CCl 4 -Cl 2
[0057] First add 60ml of carbon tetrachloride into a 100ml three-necked flask, then add 2g of off-white lithium peroxide powder, vacuumize to keep the pressure of the reactor below 100torr, stir magnetically, and then pass through a bottom with evenly distributed Chlorine gas is fed into the small-hole glass distributor for reaction, and the reactor pressure is kept at 150-300 torr. Weak red light can be observed during the reaction, and the solid powder is still suspended in carbon tetrachloride after the reaction. Point the optical fiber head coupled with the OMA-V spectrometer and the CCD spectrometer directly to the three-necked flask to monitor the radiation signal of singlet oxygen. The spectrometer can easily monitor the strong near-infrared 1270nm singlet oxygen Molecular radiation signals and weak bimolecular synergistic radiation signals at 6340nm or 703nm in the ...
Embodiment 3
[0058] Embodiment 3: sodium peroxide-carbon tetrachloride-chlorine system: Na 2 o 2 -CCl 4 -Cl 2
[0059] The reaction process is the same as in Example 2, except that the solid reactant 2g of lithium peroxide is replaced by 6g of sodium peroxide for reaction, and the reactor pressure is maintained at 150 to 300 torr. The spectrometer can easily monitor the strong 1270nm unimolecular radiation signal in the near infrared of singlet oxygen and the weak 6340nm or 703nm bimolecular synergistic radiation signal in the visible region.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com