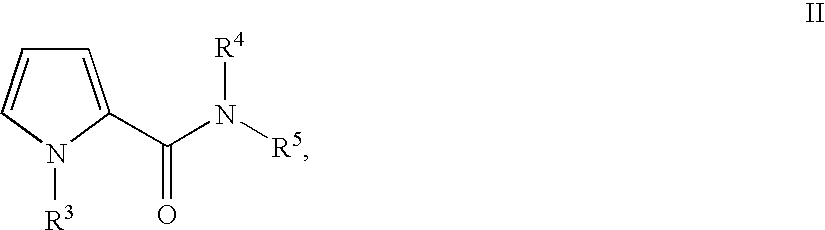Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Subjective tinnitus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Subjective tinnitus. Subjective tinnitus is the most frequent type of tinnitus. It can have many possible causes, but most commonly it results from hearing loss. When the tinnitus is caused by disorders of the inner ear or auditory nerve it is called otic (from the Greek word for ear).
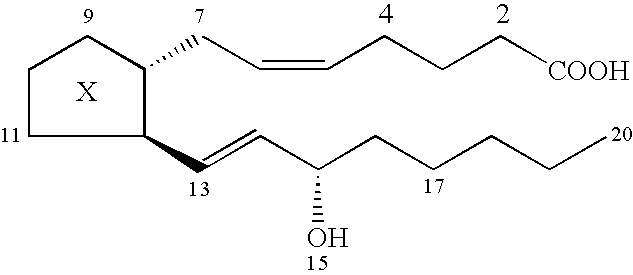
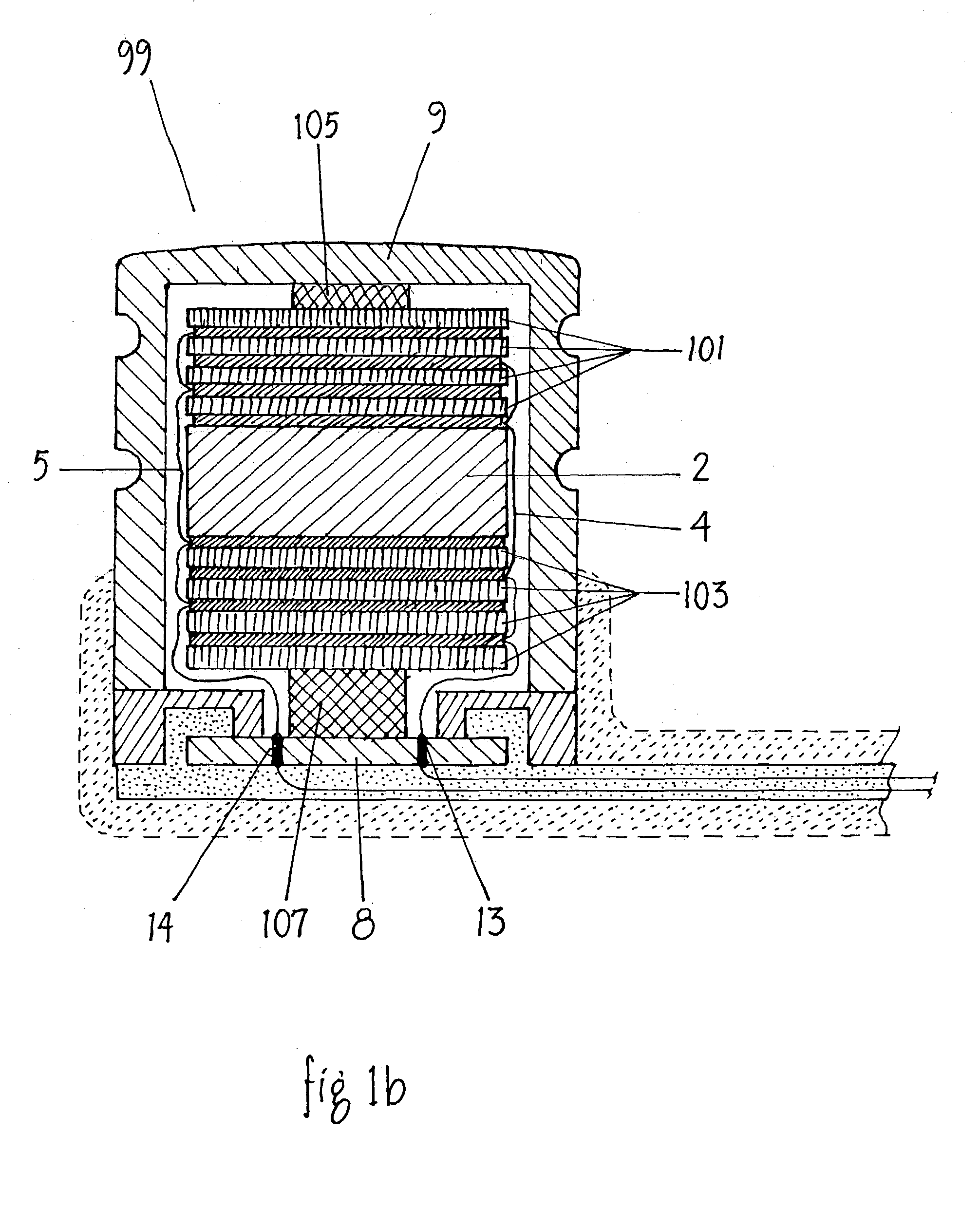
Totally implantable hearing prosthesis
ActiveUS20050020873A1Easy and safe to implantIncrease powerElectrotherapyImplantable hearing aidsCochlear implantationProsthesis
The invention comprises a totally implantable hearing prosthesis for hearing impaired persons. An inertial vibrational element is hermetically sealed and implanted in bone between the lateral and superior semicircular canals without breaching the integrity of the canals. The vibrational element is adapted to vibrate the walls of the canals and the fluids contained therein, thereby vibrating contiguous fluids within the cochlea thus stimulating hair cells and creating a hearing percept. The invention can also be adapted to be a tinnitus masking system, and / or used in combination with a coehlear implant hearing system.
Owner:MED EL ELEKTROMEDIZINISCHE GERAETE GMBH
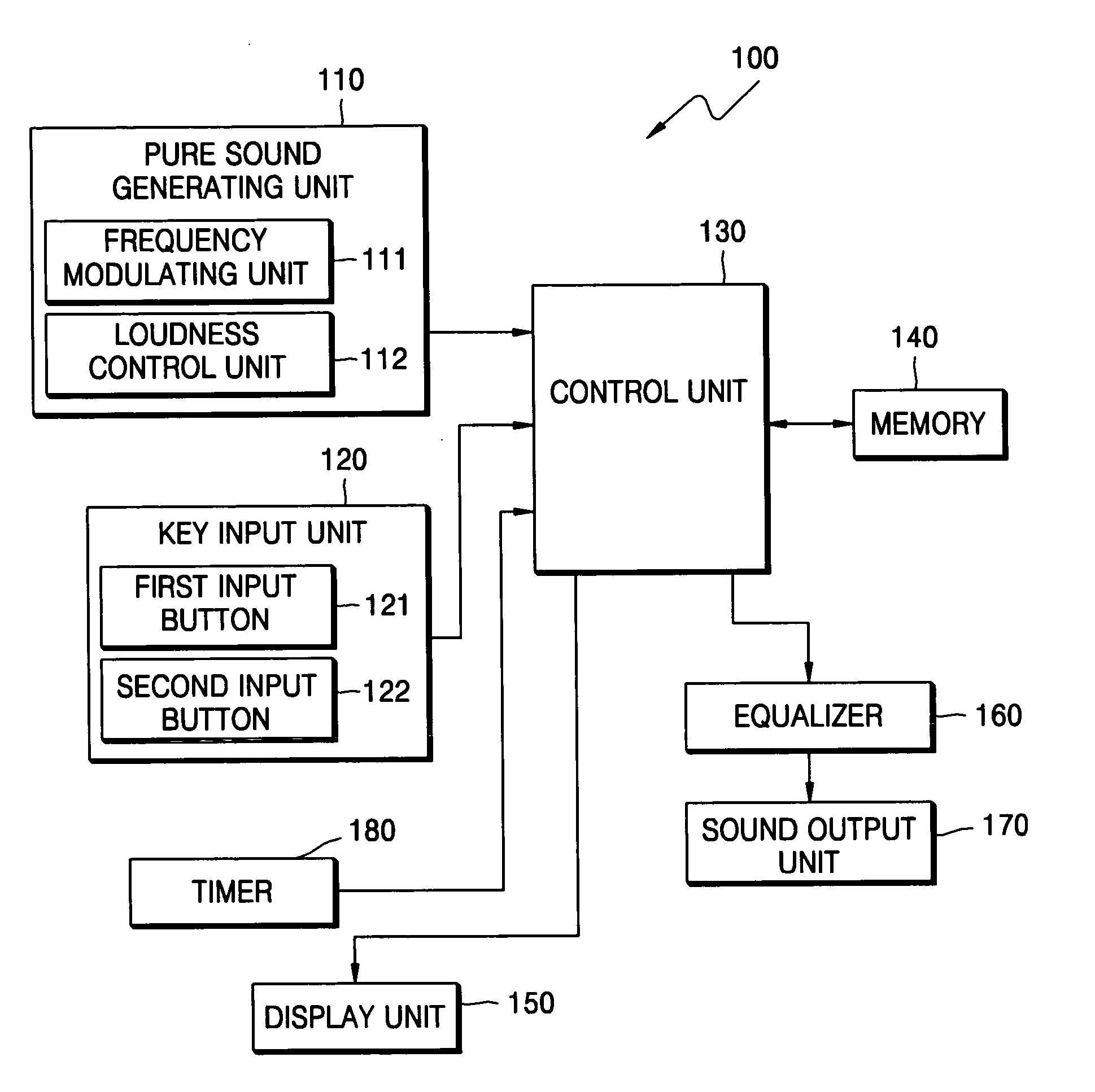
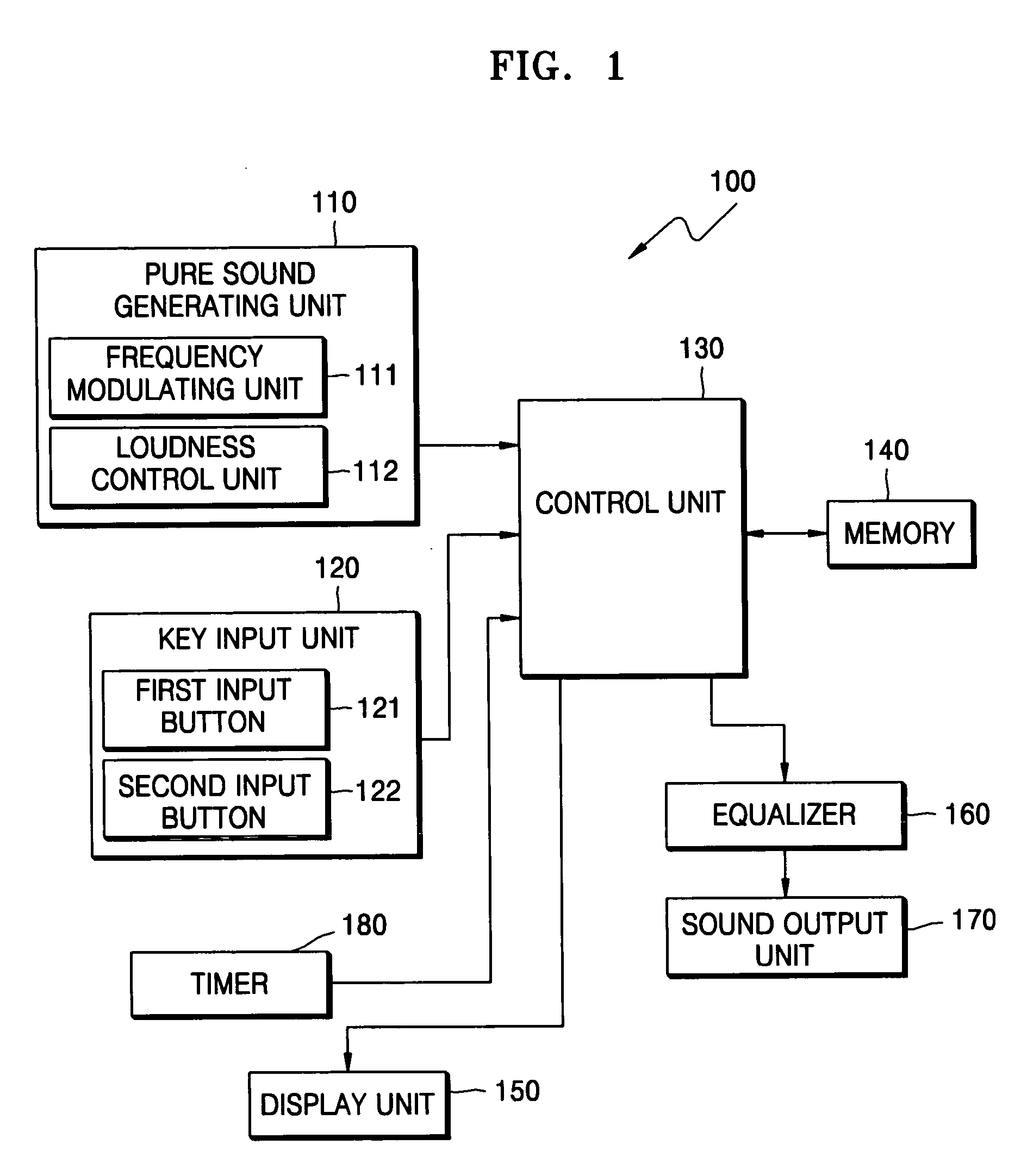
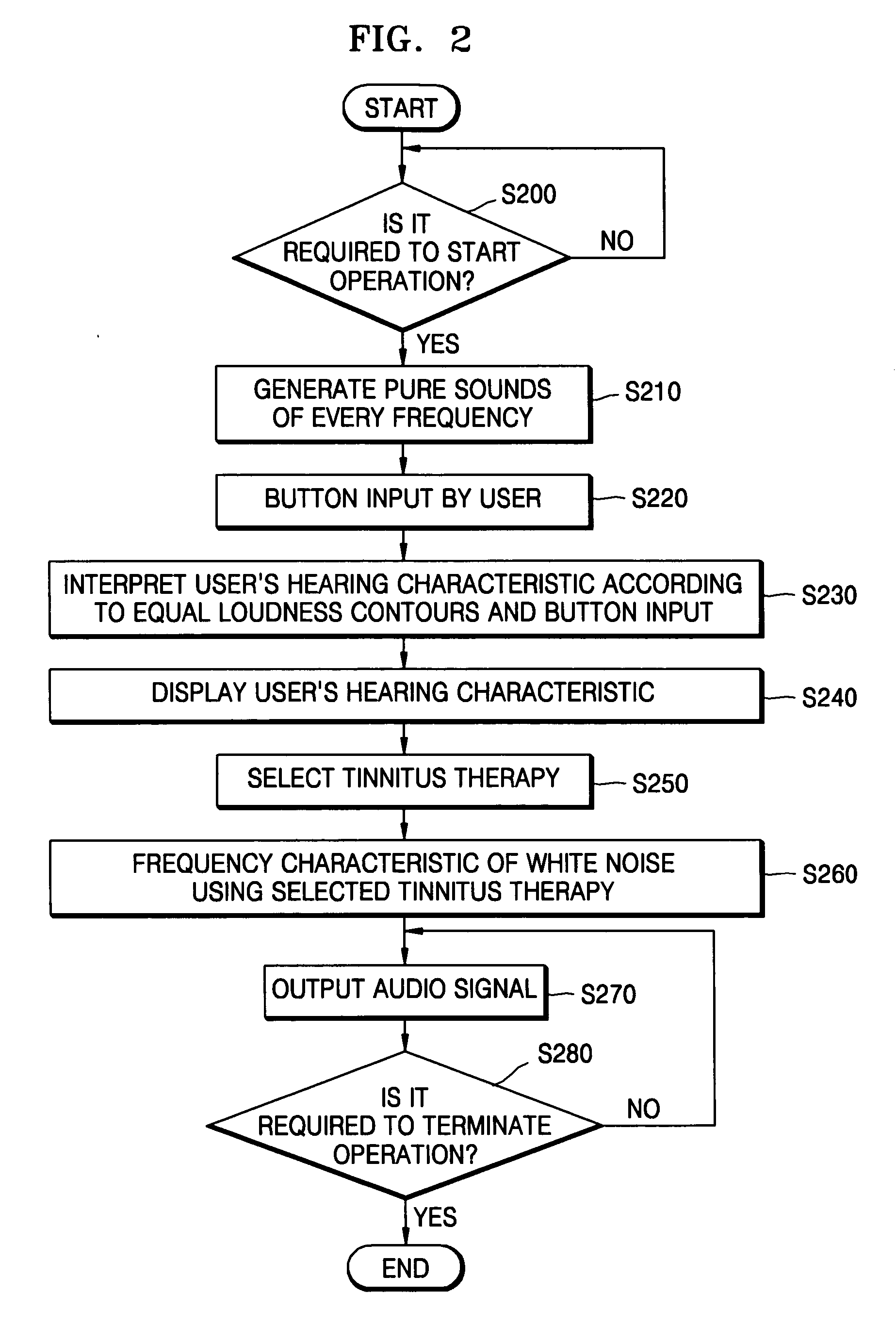
Method and device for tinnitus therapy
A method and device for tinnitus therapy. The method includes generating pure sounds, each having a predetermined frequency, within an audible range, and waiting for a user to press an input button when the user hears the pure sound. Then, the hearing characteristics of the user are interpreted in conjunction with equal loudness contours. From this interpretation, either a tinnitus masking method or a tinnitus retraining therapy are selected according to the hearing characteristics of the user.
Owner:SAMSUNG ELECTRONICS CO LTD
Systems for and methods of transcranial direct current electrical stimulation
InactiveUS20130204315A1Reduce pointsSymptoms improvedImplantable neurostimulatorsArtificial respirationElectricityNervous system
A system according to the present invention provides a portable, non-invasive device adapted to deliver electrical stimulation to a brain, such as to treat tinnitus. Such system is preferably a head-worn system configured to provide transcranial direct current electrical stimulation (tDCS) to a patient, where a therapy based at least partially thereon may be self-administered by the patient. tDCS is a non-invasive method of brain stimulation to treat tinnitus, or other neurological indications, that may provide significant relief. Methods according to the present invention include preferably brief sessions of anodal tDCS to assist in determining adequate electrode location and stimulus intensity by producing transient decreases in tinnitus intensity. Methods may also or alternatively include a number of sessions of cathodal tDCS at a confirmed electrode location and stimulus intensity to provide sustained tinnitus relief. Methods may also or alternatively include a number of maintenance sessions to prolong the sustained relief.
Owner:NDI MEDICAL
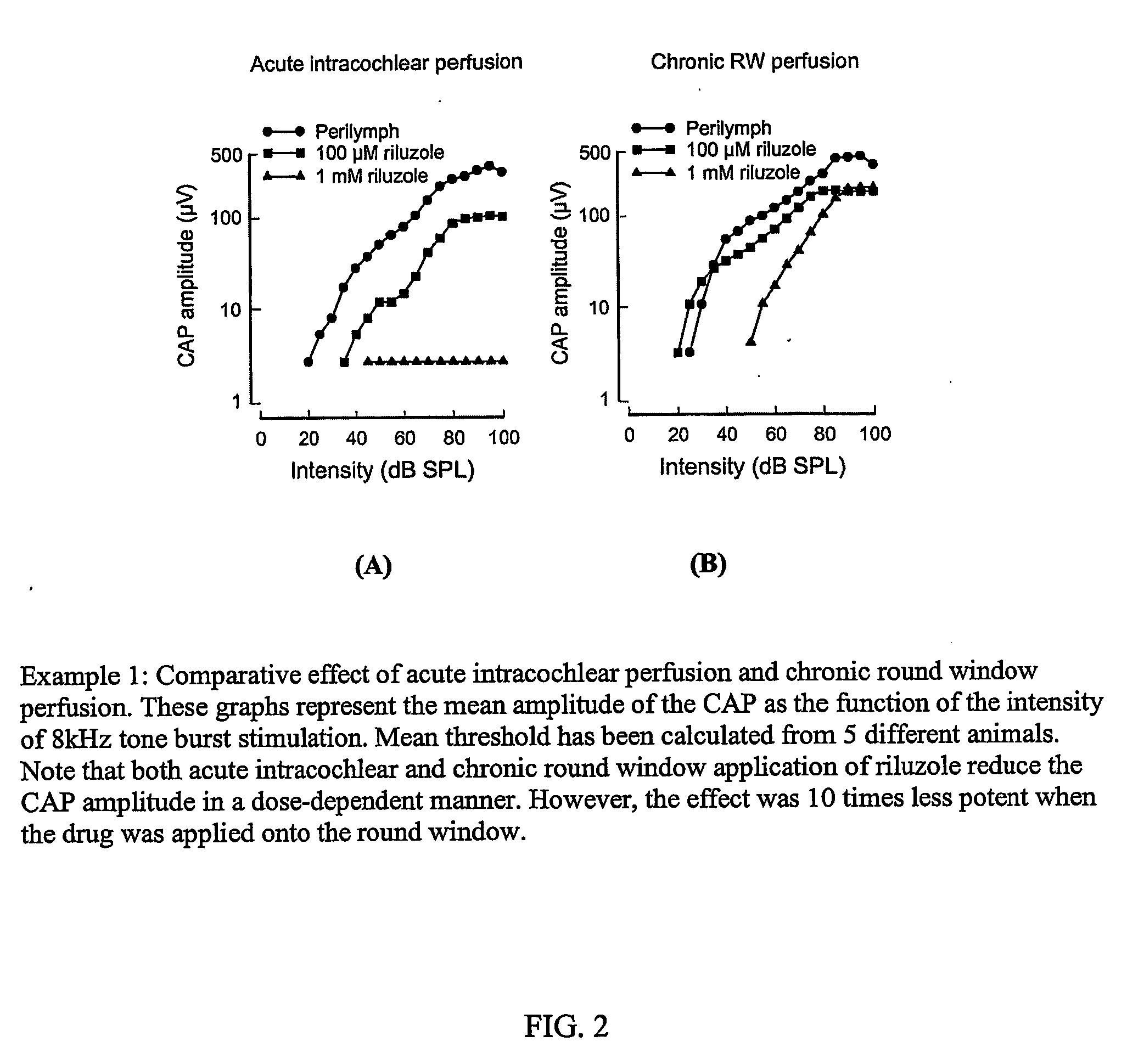
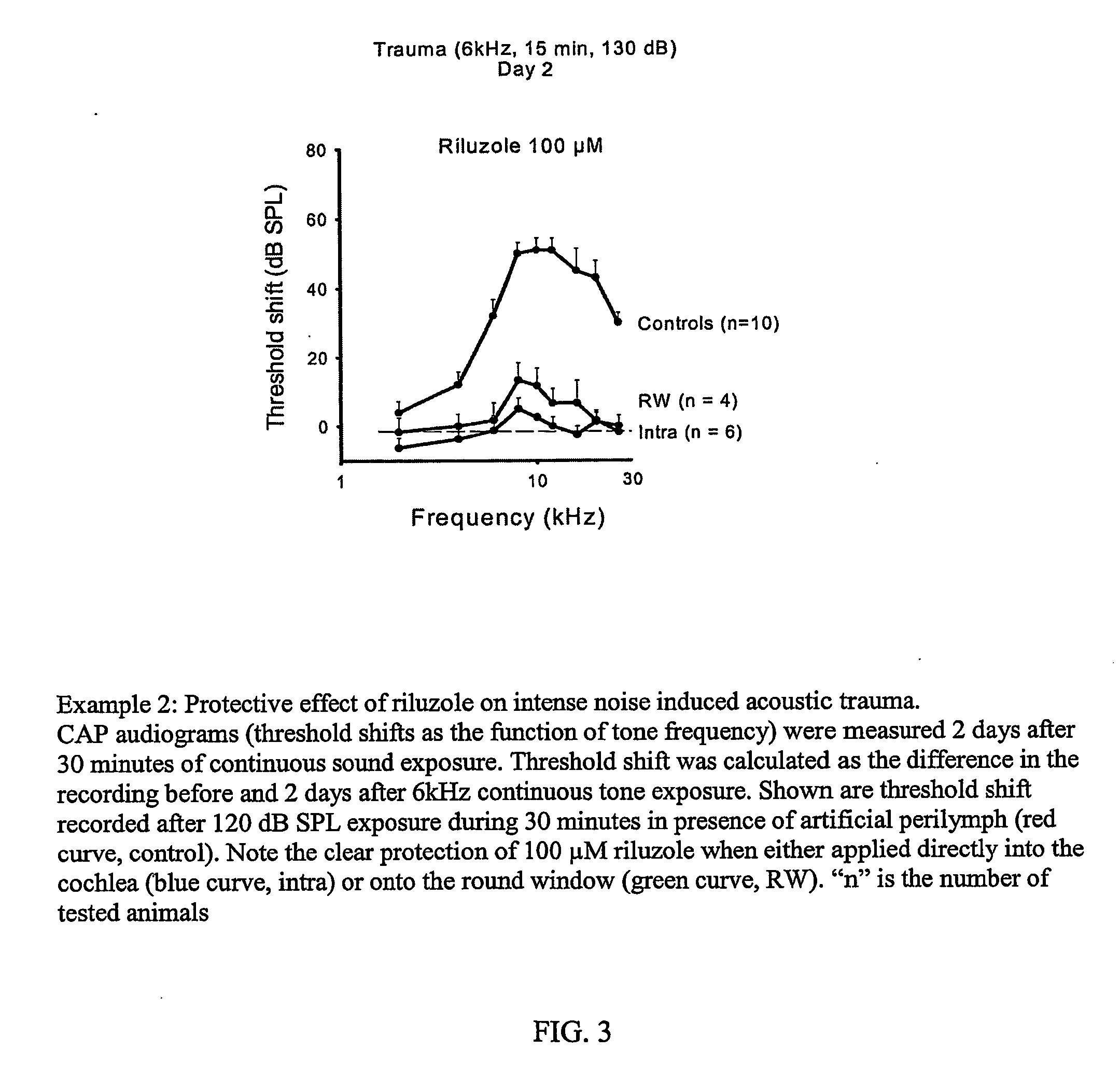
Methods for the treatment of tinnitus induced by cochlear excitotoxicity
InactiveUS20060063802A1Suppress and reduce NMDA receptor mediated aberrant activityPreventing and treating tinnitusCompounds screening/testingBiocideNR1 NMDA receptorTotal Deafness
The invention relates to methods for the prevention and / or treatment of tinnitus induced by cochlear excitotoxicity. In these methods, a pharmaceutical composition comprising an NMDA receptor antagonist is administered to an individual in need of such treatment by appropriate devices and / or formulations for local administration to the inner ear. The tinnitus to be prevented and / or treated may be provoked by acoustic trauma, presbycusis, ischemia, anoxia, treatment with one or more ototoxic medications, sudden deafness, or other cochlear excitotoxic-inducing occurrence. The invention also relates to method for the identification of compounds effective in the treatment and prevention of tinnitus by a novel screening method incorporating an electrophysiological test method.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Aural rehabilitation system and a method of using the same
InactiveUS20060029912A1ElectrotherapyCharacter and pattern recognitionAutomatic controlSubjective tinnitus
A system 2 and method 64 for neurological rehabilitation or training is disclosed. The system 2 can be used to improve listening, comprehension, and communication. The system 2 can be controlled automatically by a remote device 6 or manually by a physician's device 4. The system 2 can store data in, and retrieve data from a database 10 for analysis, reporting and execution. The system 2 can adapt and adjust based on the subject's performance. The system 2 can be used to treat hearing loss, tinnitus or other audiological health problems.
Owner:NEUROTONE
Methods for the treatment of tinnitus induced by cochlear excitotoxicity
ActiveUS20050214338A1Suppress and reduce NMDA receptor mediated aberrant activityPreventing and treating tinnitusBiocideHalogenated hydrocarbon active ingredientsNR1 NMDA receptorTotal Deafness
The invention relates to methods for the prevention and / or treatment of tinnitus induced by cochlear excitotoxicity. In these methods, a pharmaceutical composition comprising an NMDA receptor antagonist is administered to an individual in need of such treatment by appropriate devices and / or formulations for local administration to the inner ear. The tinnitus to be prevented and / or treated may be provoked by acoustic trauma, presbycusis, ischemia, anoxia, sudden deafness, or other cochlear excitotoxic-inducing occurrence.
Owner:AURIS MEDICAL AG
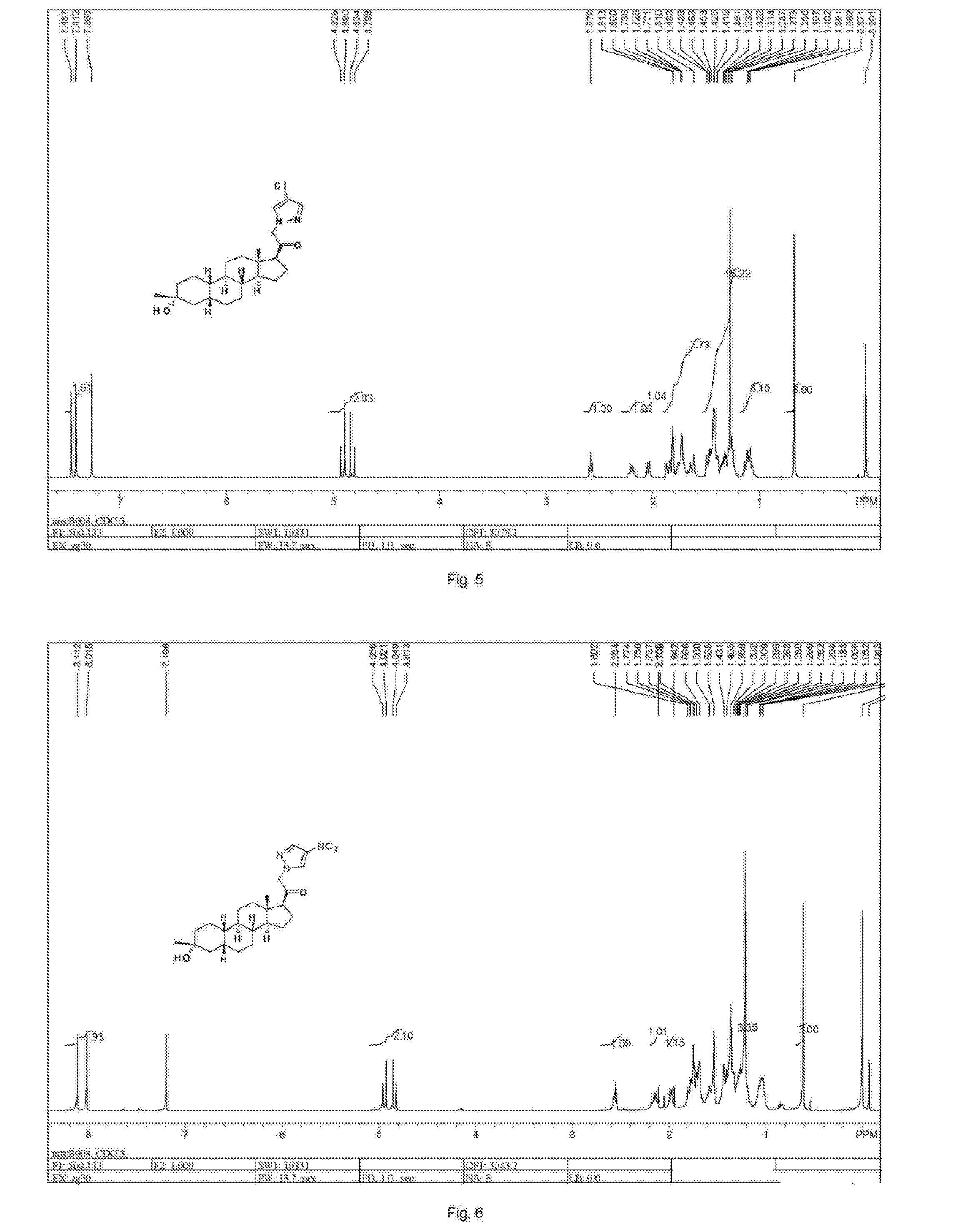
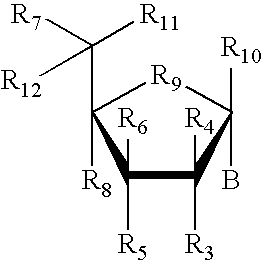
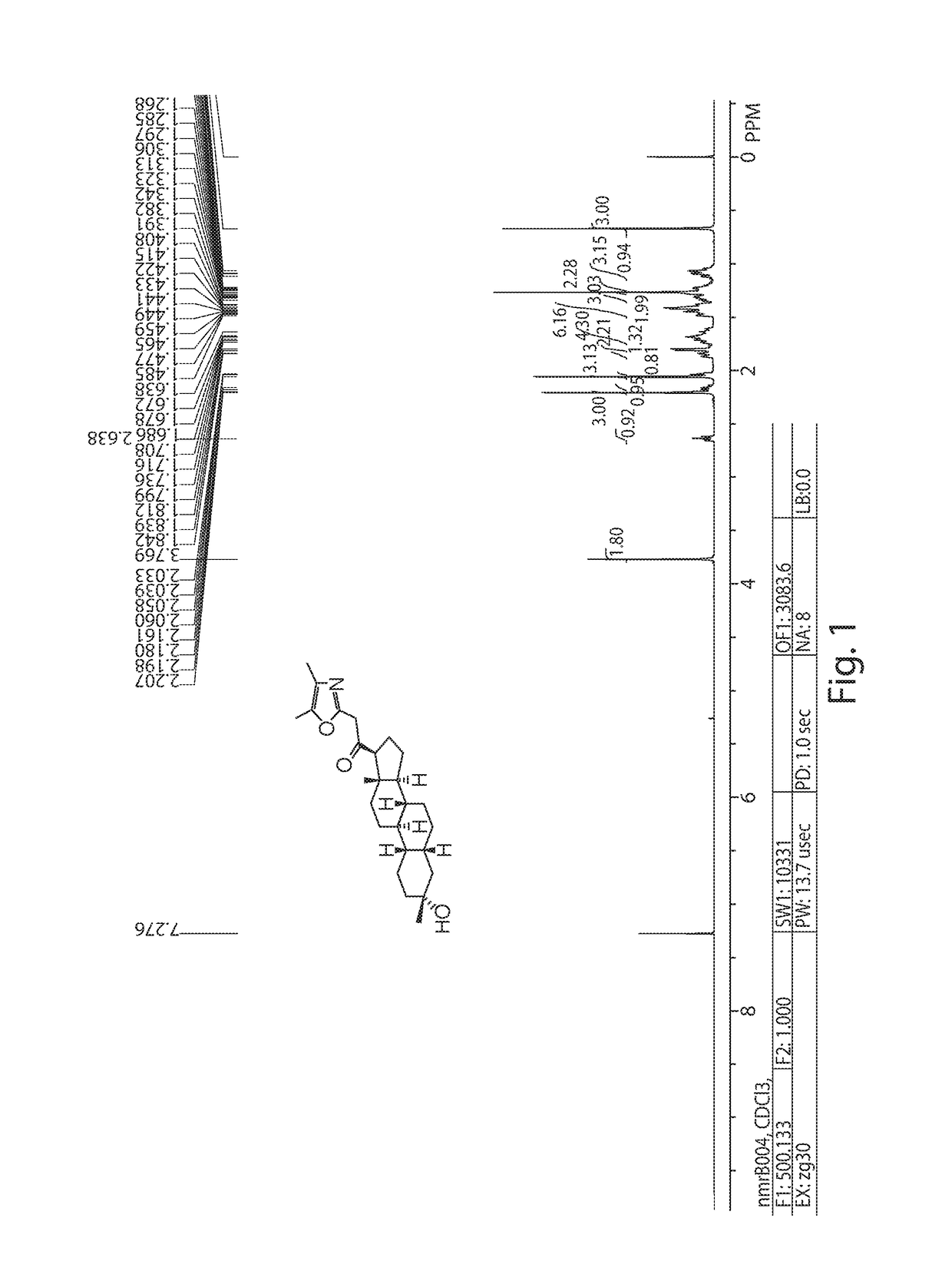
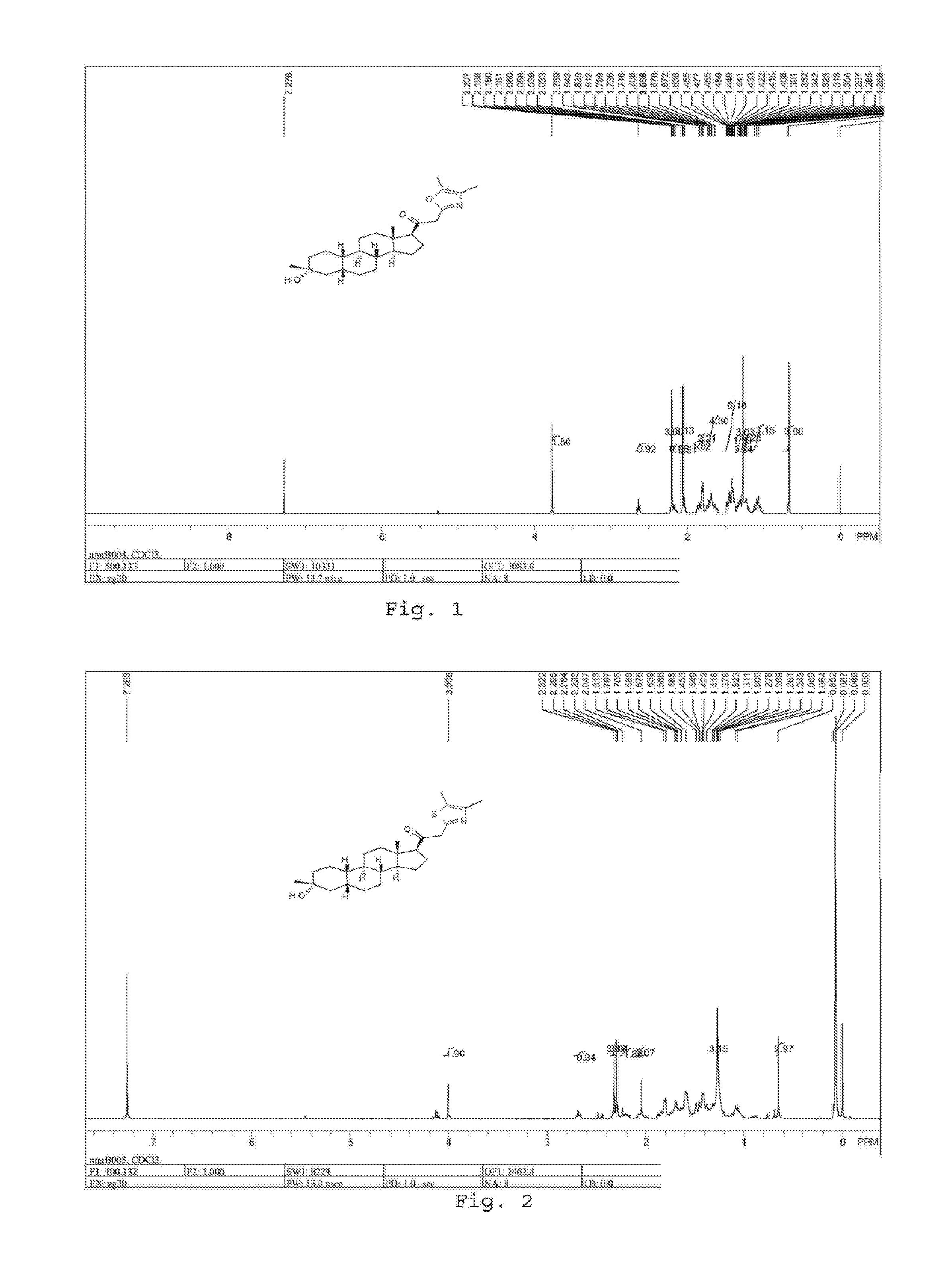
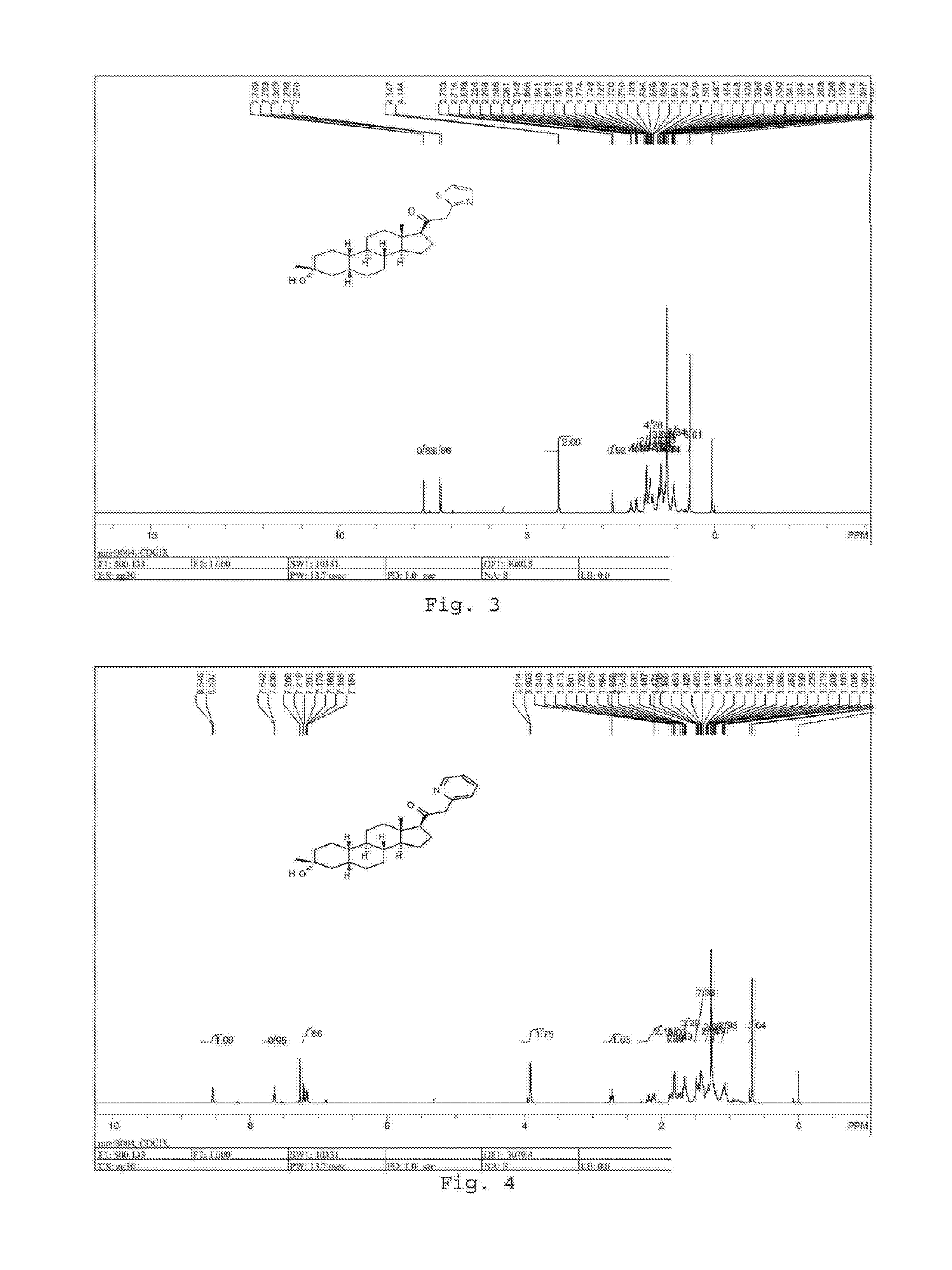
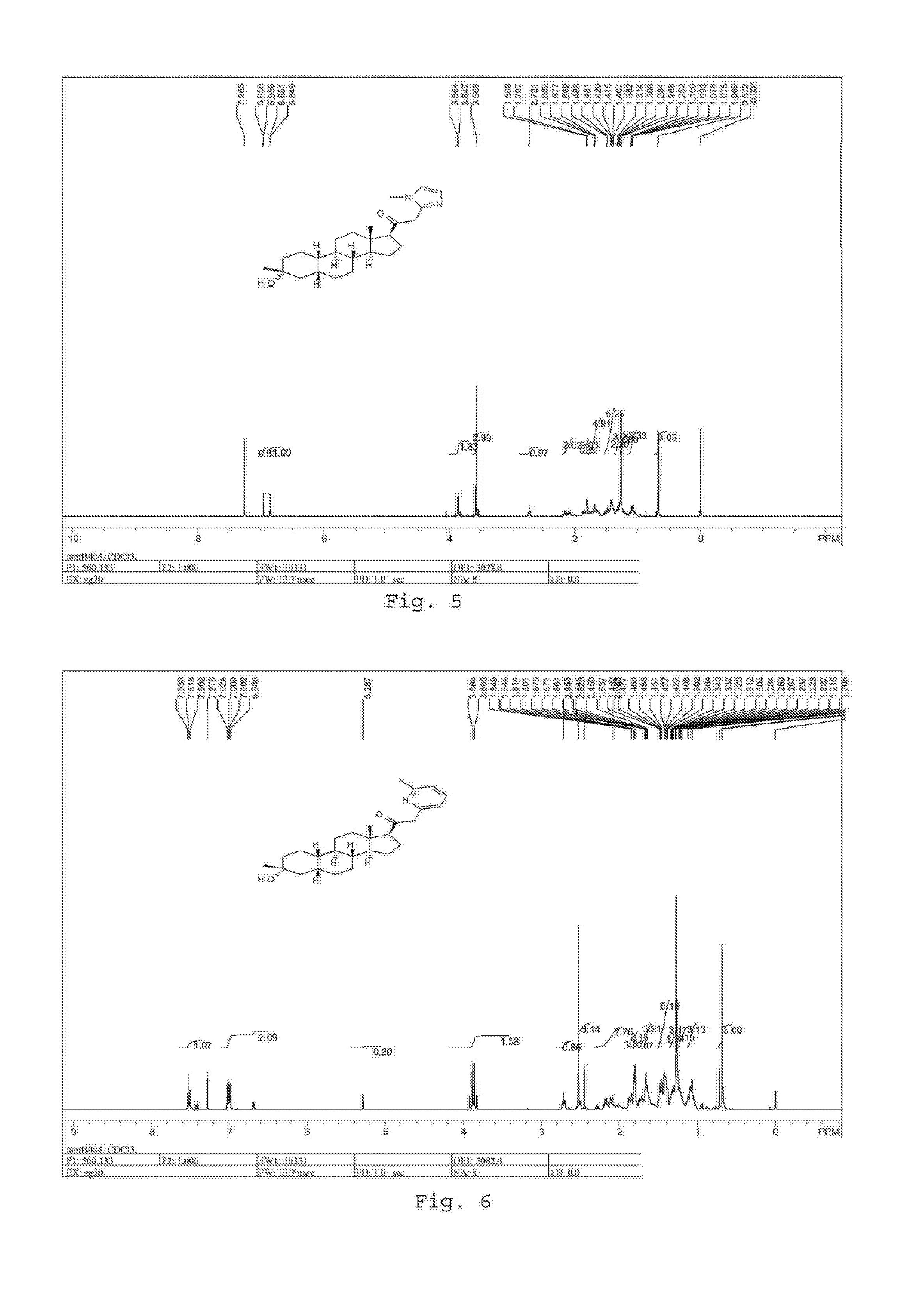
19-nor C3, 3-disubstituted C21-N-pyrazolyl steroids and methods of use thereof
ActiveUS9512165B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderSubstance abuserWithdrawal syndrome
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
19-nor c3, 3-disubstituted c21-n-pyrazolyl steroids and methods of use thereof
ActiveUS20160108080A1Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsSenses disorderWithdrawal syndromeSubstance abuse disorder
Provided herein are 19-nor C3,3-disubstituted C21-pyrazolyl steroids of Formula (I), and pharmaceutically acceptable salts thereof; wherein-, R1, R2, R3a, R3b, R4a, R4b, R5, R6, and R7 are as defined herein. Such compounds are contemplated useful NI for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
RNA interference mediated inhibition of retinolblastoma (RBI) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050260620A1Improve bioavailabilityMinimize the possibilitySugar derivativesMicrobiological testing/measurementBalance disturbancesSubjective tinnitus
This invention relates to compounds, compositions, and methods useful for modulating retinoblastoma (RB1) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of retinoblastoma gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of retinoblastoma genes. Such small nucleic acid molecules are useful, for example, for treating, preventing, inhibiting, or reducing hearing loss, deafness, tinnitus, motion and balance disorders, cancer and proliferative diseases and any other disease, condition, trait or indication that can respond to the level of retinoblastoma in a cell or tissue, alone or in combination with other treatments or therapies.
Owner:SIRNA THERAPEUTICS INC
19-nor C3, 3-disubstituted C21-C-bound heteroaryl steroids and methods of use thereof
ActiveUS9725481B2Eliminate potential for oxidationImprove bioavailabilityOrganic active ingredientsNervous disorderSubstance abuserWithdrawal syndrome
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5-to6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
Prevention and treatment of hearing disorders
Compositions, and methods of use thereof, are provided for the prevention, treatment or alleviation of symptoms of hearing are provided. Embodiments of the methods employ zonisamide as the sole active pharmaceutical agent or a combination of zonisamide and another pharmaceutical agent, such as an antioxidant, a NMDA antagonist, an SSRI or a combined SSRI / NMDA antagonist agent. Other embodiments of the method involve the use of zonisamide alone or in combination with another API to prevent, treat or ameliorate one or more symptoms of hearing loss. Hearing disorders treatable with the invention include noise-induced hearing loss, drug-induced hearing loss, central auditory hearing disorder (CAPD), tinnitus and presbyacusis.
Owner:CYPRESS BIOSCI
Implantable medical devices with multiple transducers
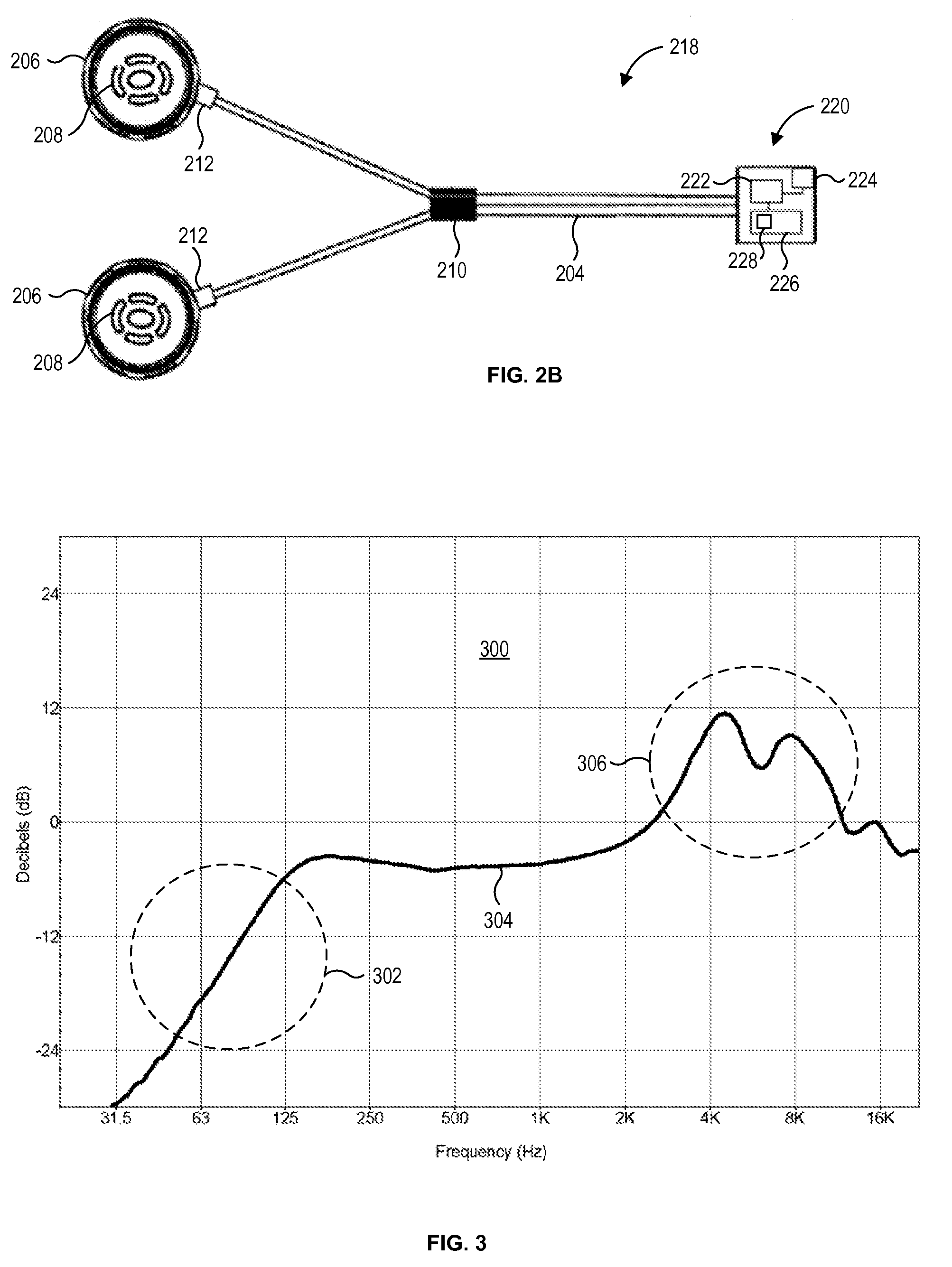
ActiveUS20070282397A1Improved sound perceptionSimple structureHead electrodesIntravenous devicesSound perceptionTransducer
The present invention relates to implantable medical devices for improving sound perception by individuals with severe to profound hearing loss or tinnitus. In particular, the present invention provides methods and devices for stimulating structures of the ear via multiple signal transducers.
Owner:MED EL ELEKTROMEDIZINISCHE GERAETE GMBH
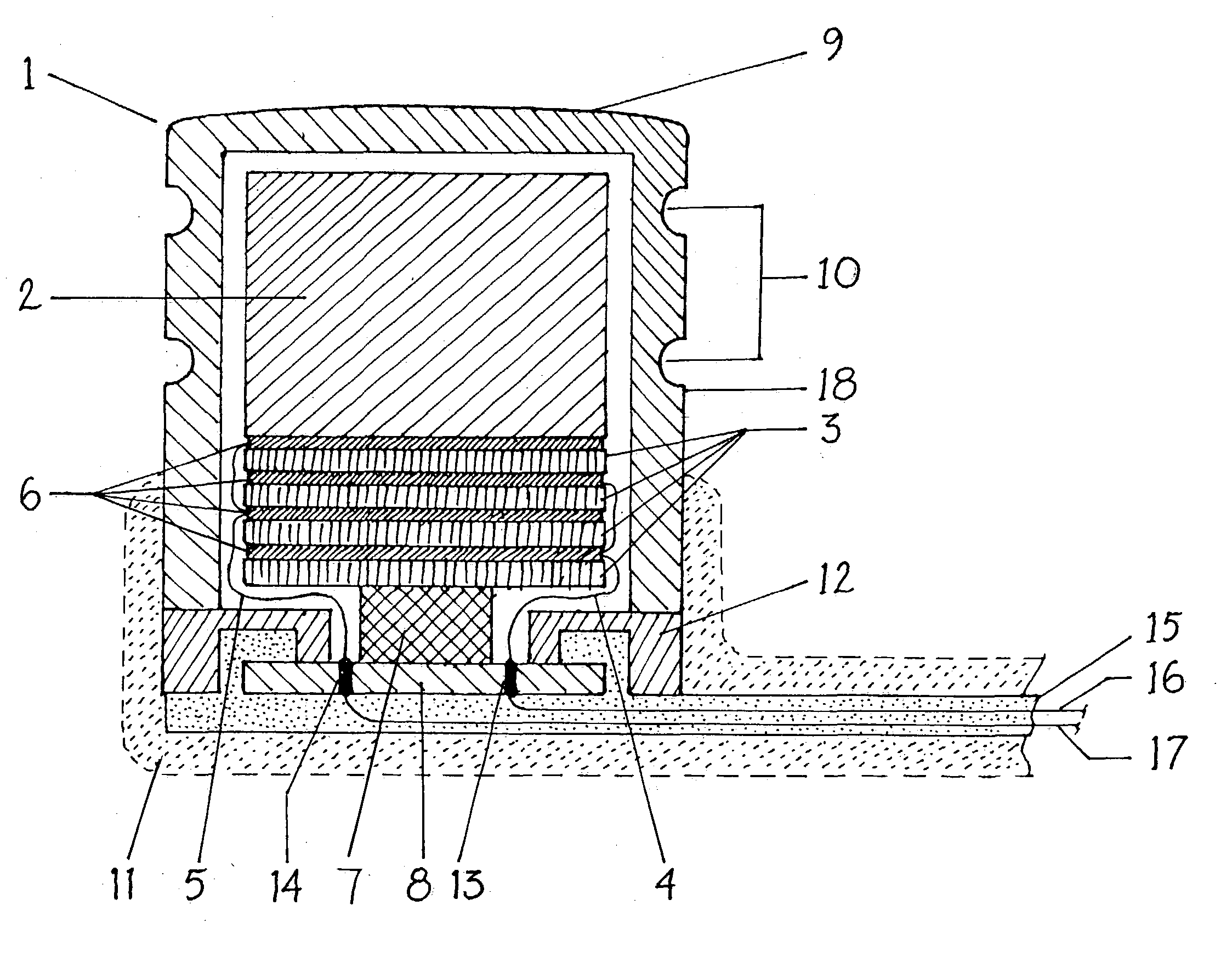
Endosteal electrode
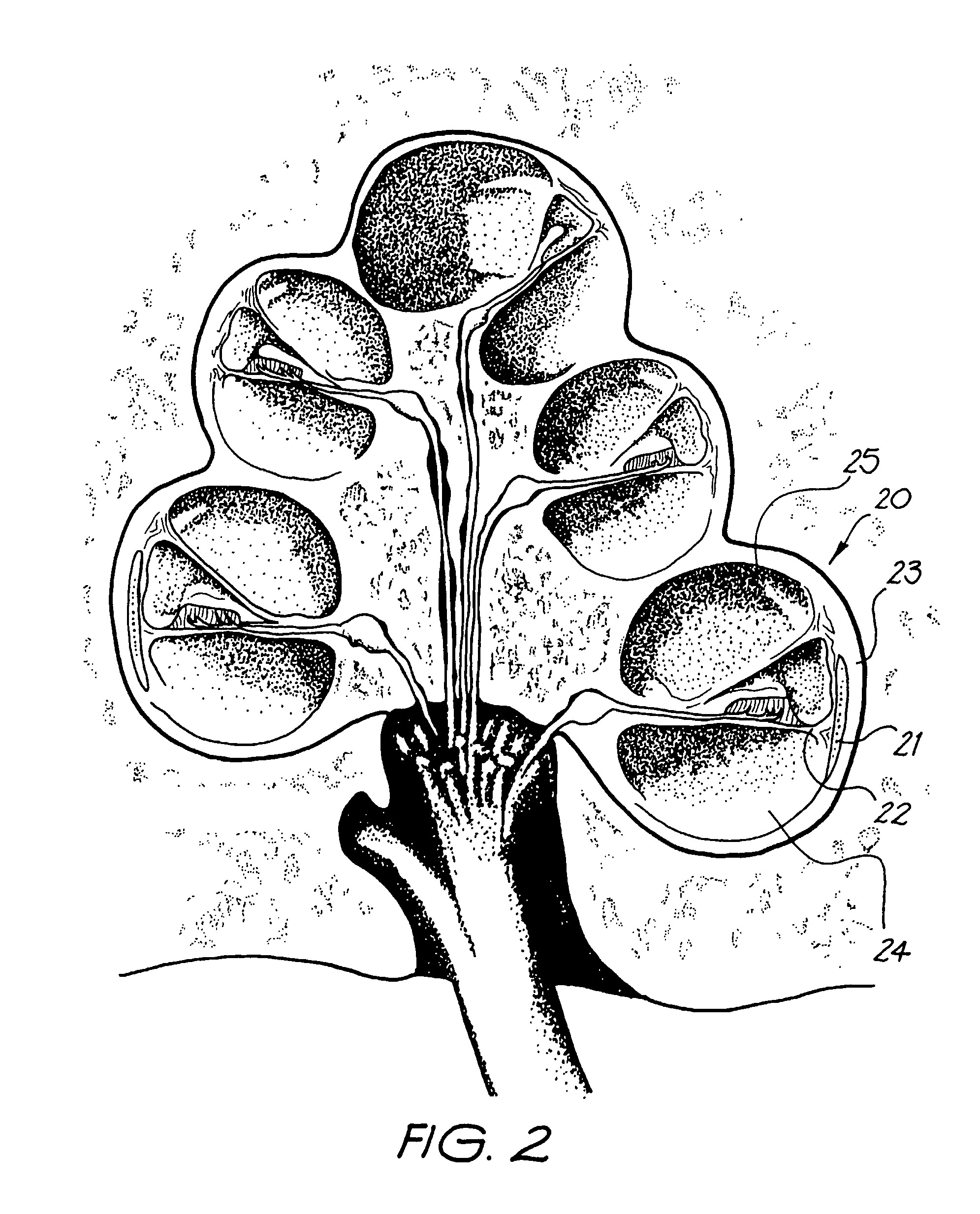
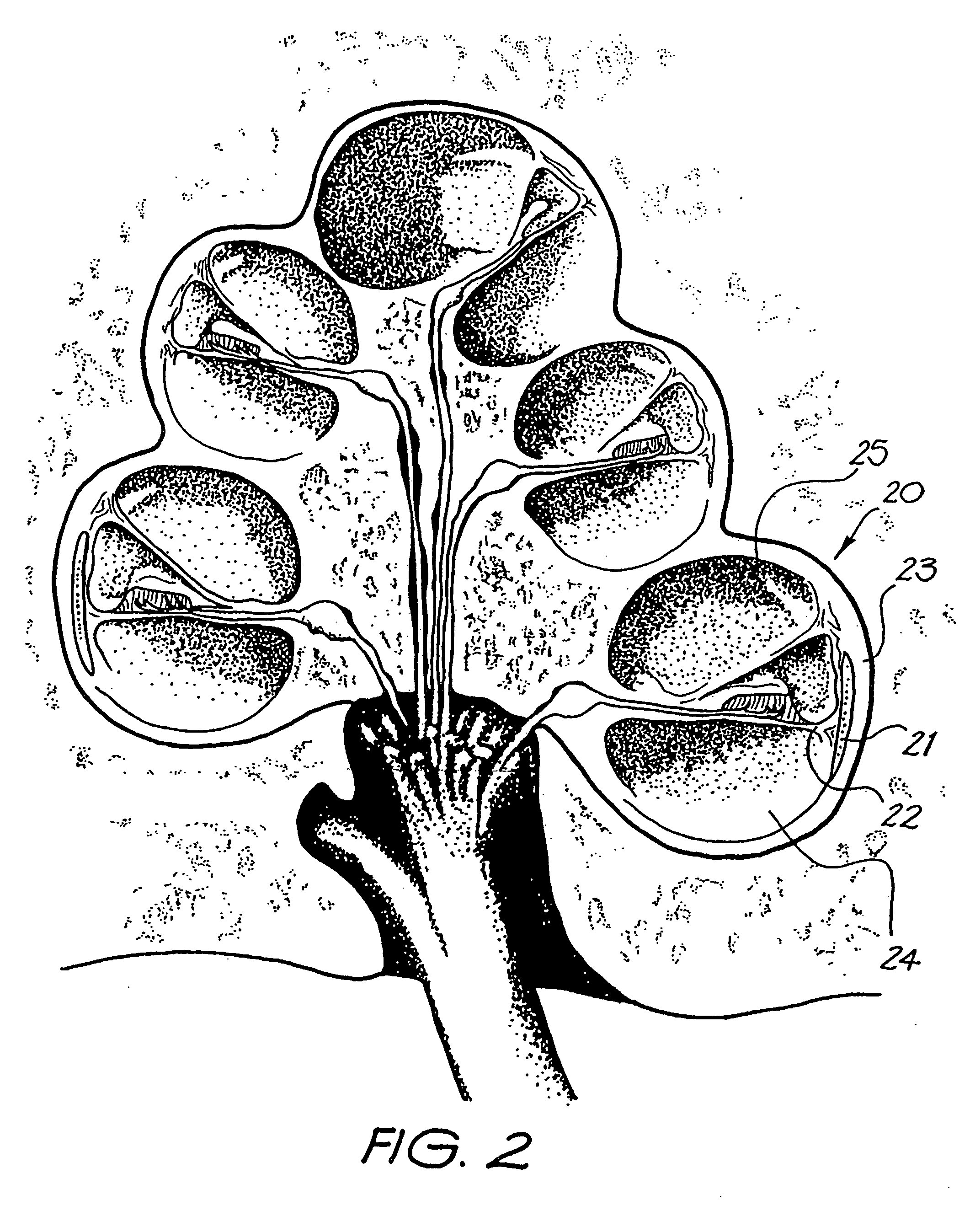
InactiveUS20050080473A1Improve hearingMaximises possibilityHead electrodesEar treatmentCochlear implantationEngineering
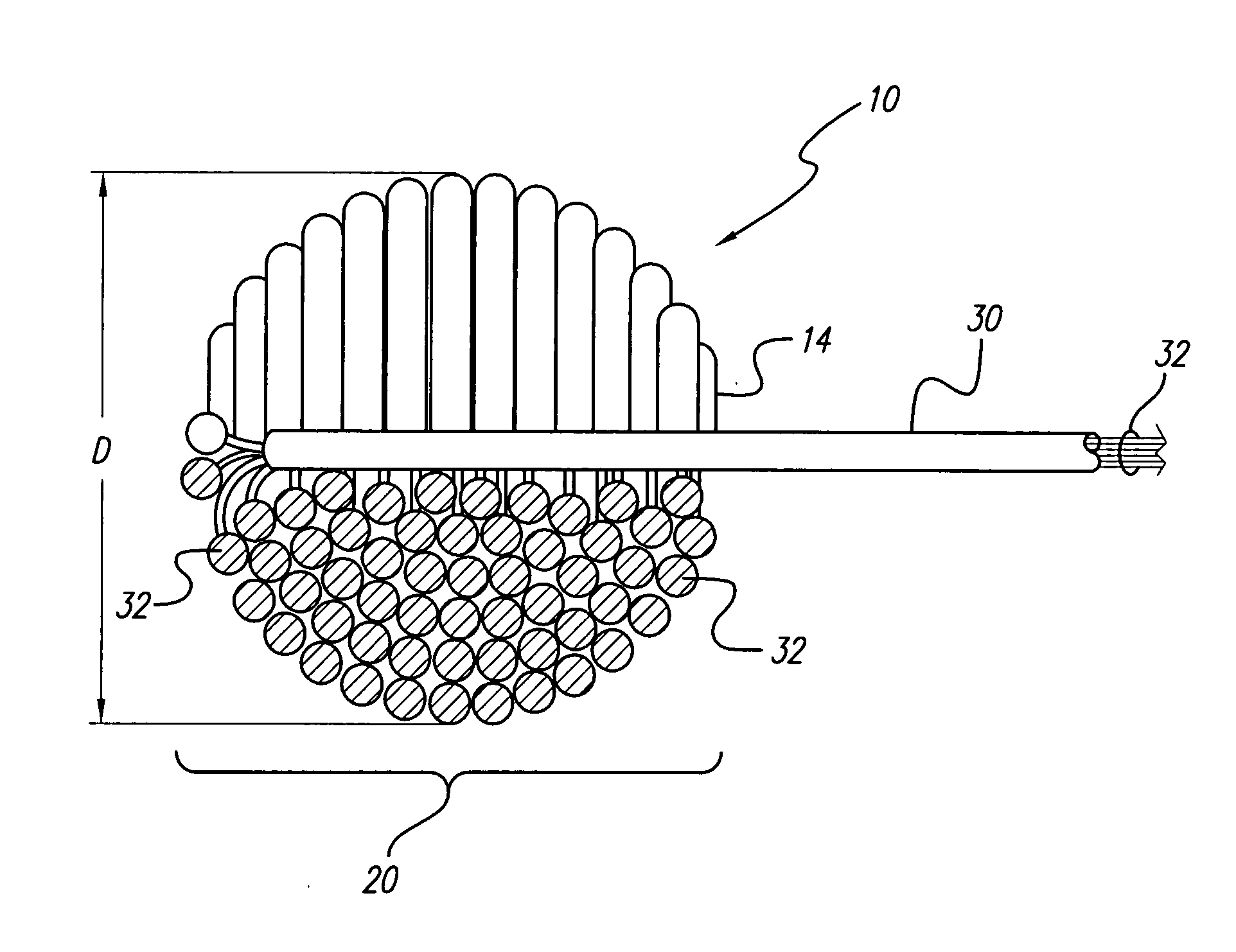
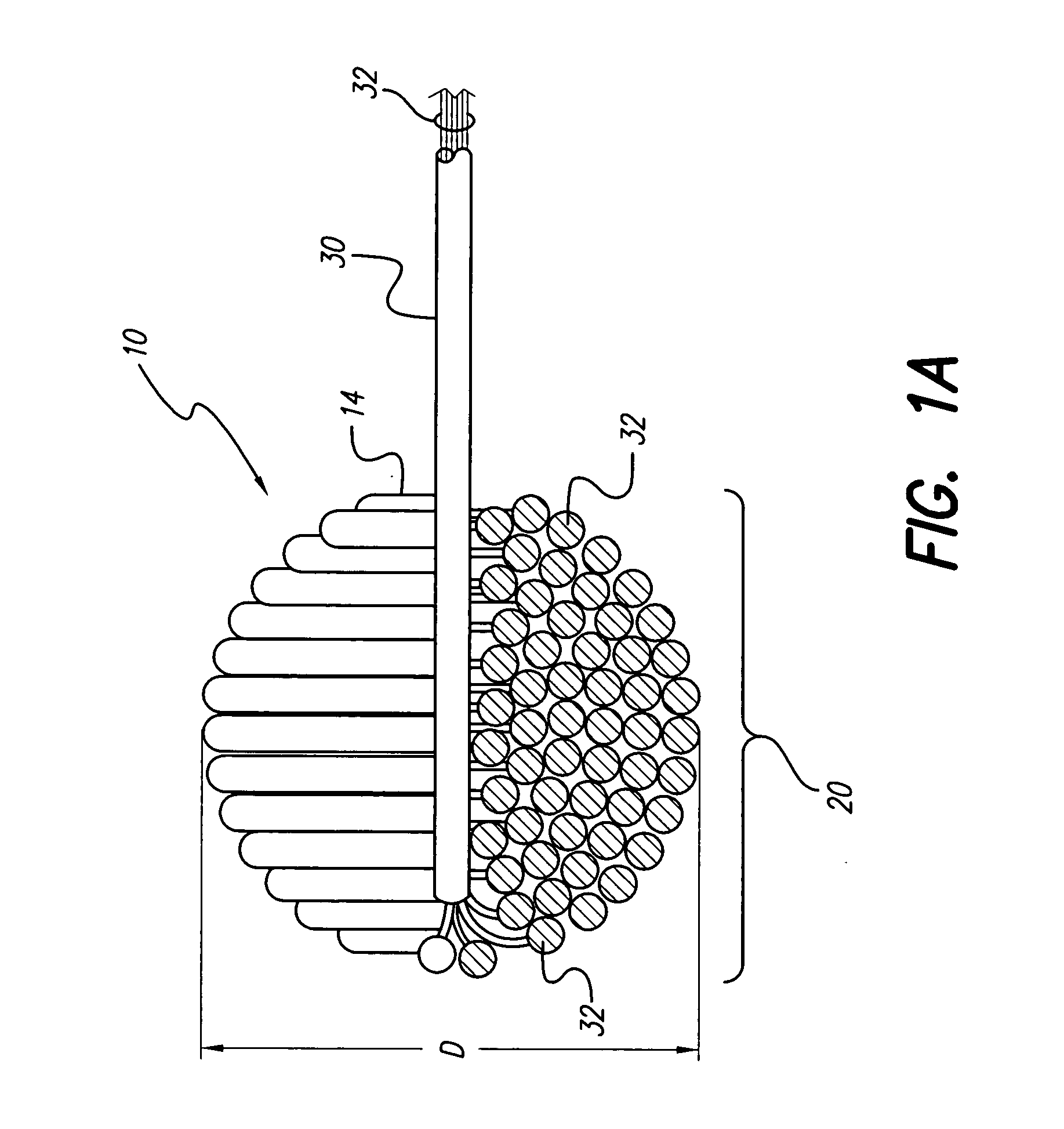
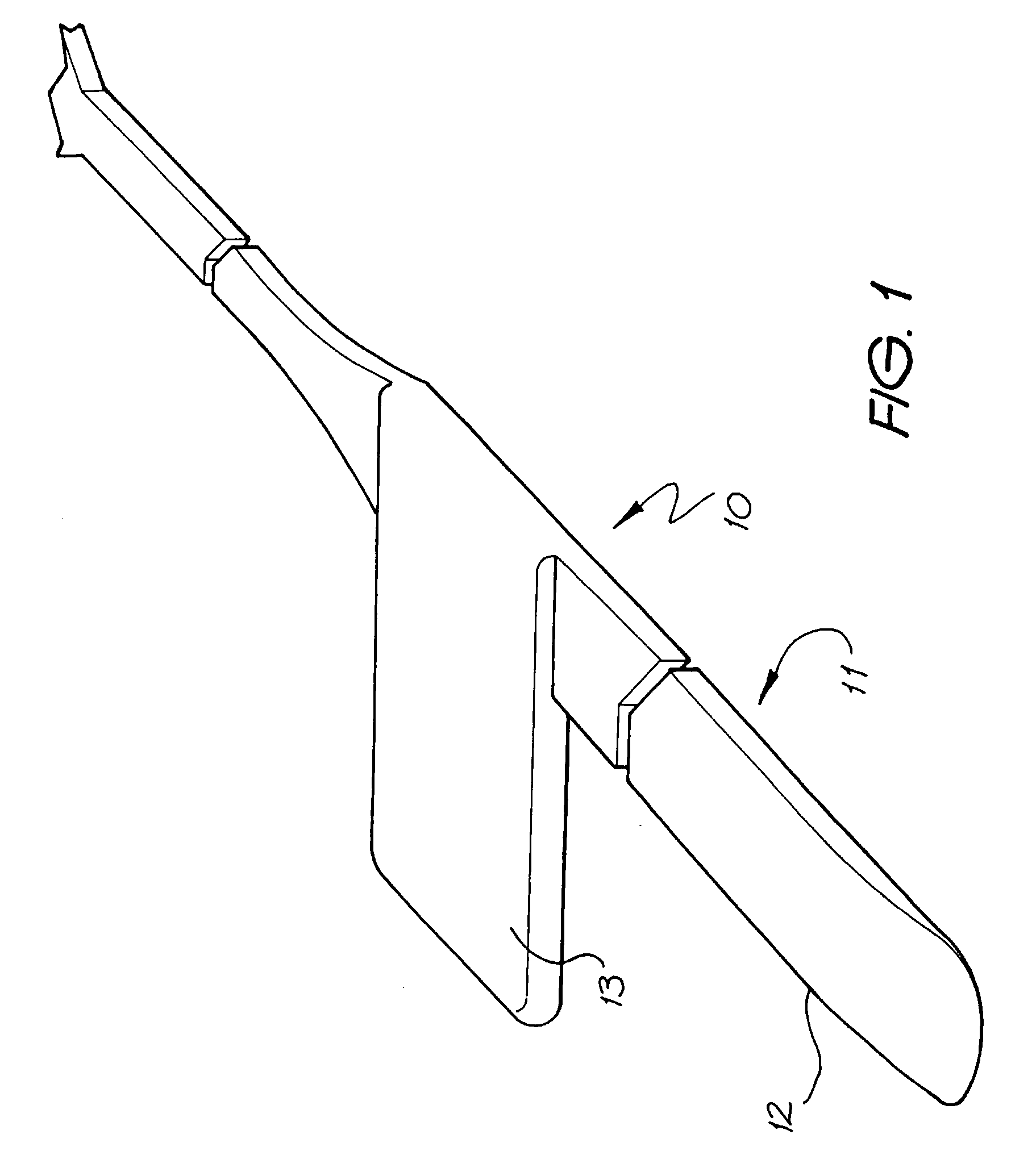
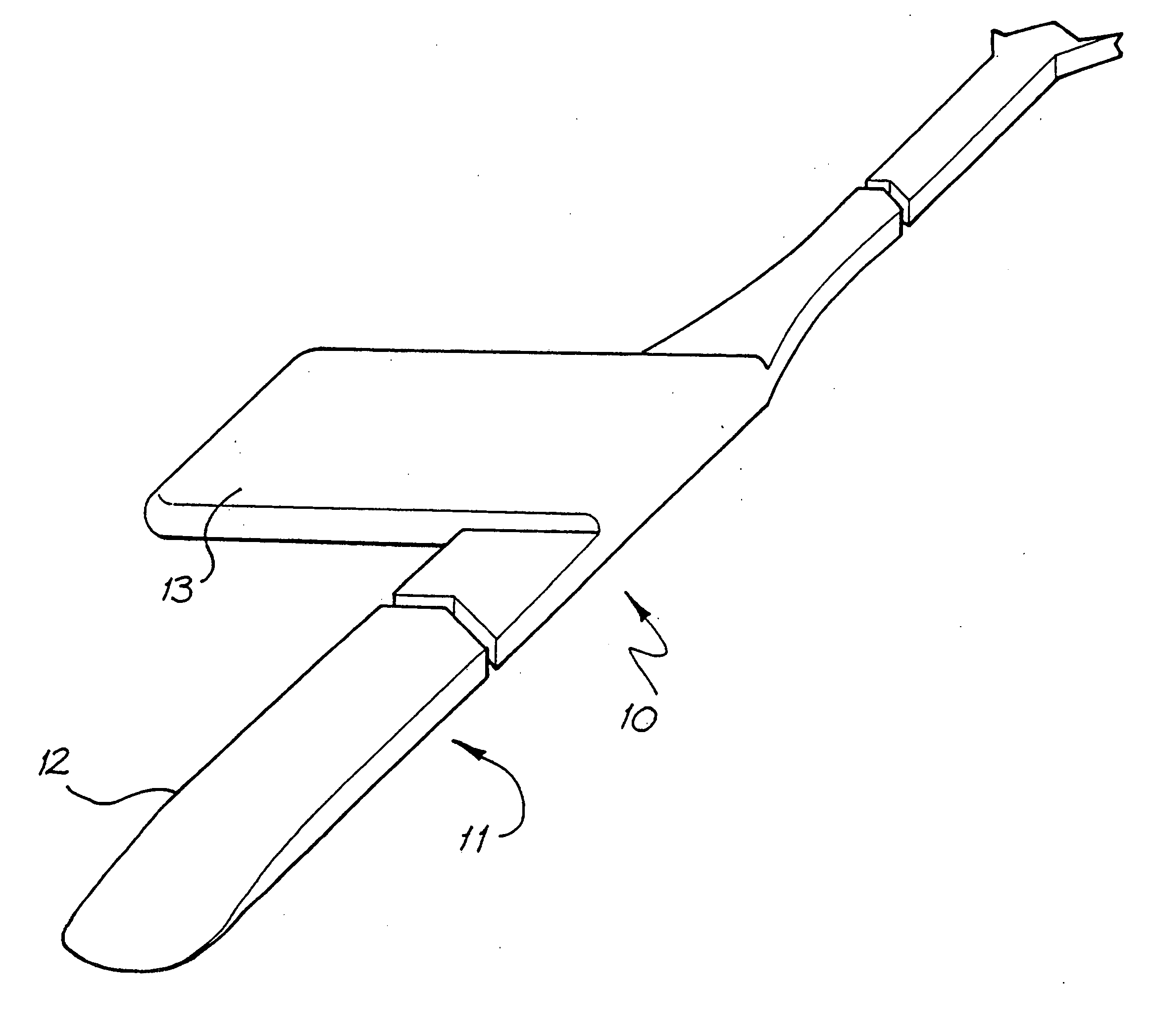
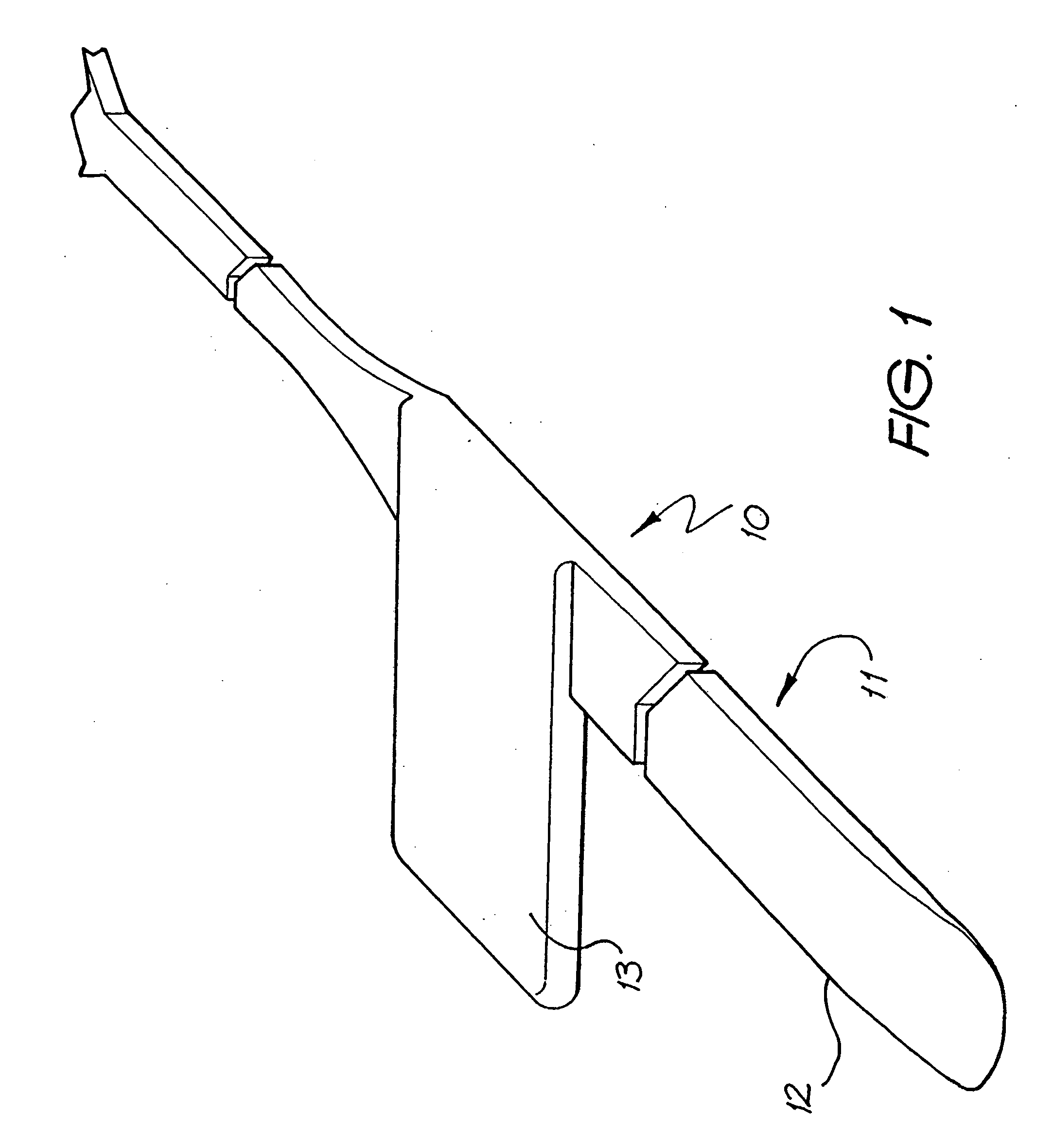
An implantable tissue-stimulating device comprising an elongate electrode carrier member (11) having a plurality of electrodes thereon. The electrodes are preferably disposed in a linear array on the carrier member (11) and are adapted to apply a preselected tissue stimulation to the cochlea. The carrier member (11) is preformed from a resiliently flexible biocompatible silicone and extends from a distal end (12) to a stop member (13). The carrier member (11) is adapted for intracochlear but extraluminar insertion within the cochlea of an implantee. In particular, the carrier member (11) is adapted to be implanted in the crevice (21) between the spiral ligament (22) and the endosteum (23) of the lateral wall of the cochlea (20). This is a quite different location to the normal placement of the cochlear implant electrode array in the scala tympani (24) of the cochlea (20). The placement of the carrier member (11) is designed to avoid any breach of the internal ducts of the cochlea (20), such as the scala tympani (24) and scala vestibuli (25) so that the normal hydrodynamic behaviour of the cochlea (20) is not affected by any intrusive device. By preservng the normal hydrodynamic behaviour of the cochlea (20), use of the carrier member (11) maximises the possibility of also preserving any hearing of the implantee that is offered by the cochlea (20). Use of the device in a system for masking or treating the symptoms of tinnitus is also described.
Owner:COCHLEAR LIMITED
Tinnitus treatment device
A tinnitus treatment device includes a sound generation device and a receiver unit connected to the sound generation device. The receiver unit is positioned in an open-ear configuration within the ear canal of a user and is dimensioned so as to reduce insertion loss and / or occlusion effects. The sound generation device is located in a housing positioned behind the user's ear.
Owner:BAUMAN NATAN
19-nor c3, 3-disubstituted c21-c-bound heteroaryl steroids and methods of use thereof
ActiveUS20160083417A1Eliminate potential for oxidationInhibit metabolismOrganic active ingredientsNervous disorderDiseaseSubstance abuser
Provided herein are 19-nor C3,3-disubstituted steroids of Formula (I): and pharmaceutically acceptable salts thereof; wherein, , R1, R2, R3a, R3b, R4a, and R4b are as defined herein, and A is a carbon bound substituted or unsubstituted 5- to 6-membered heteroaryl ring as defined herein. Such compounds are contemplated useful for the prevention and treatment of a variety of CNS-related conditions, for example, treatment of sleep disorders, mood disorders, schizophrenia spectrum disorders, convulsive disorders, disorders of memory and / or cognition, movement disorders, personality disorders, autism spectrum disorders, pain, traumatic brain injury, vascular diseases, substance abuse disorders and / or withdrawal syndromes, and tinnitus.
Owner:SAGE THERAPEUTICS
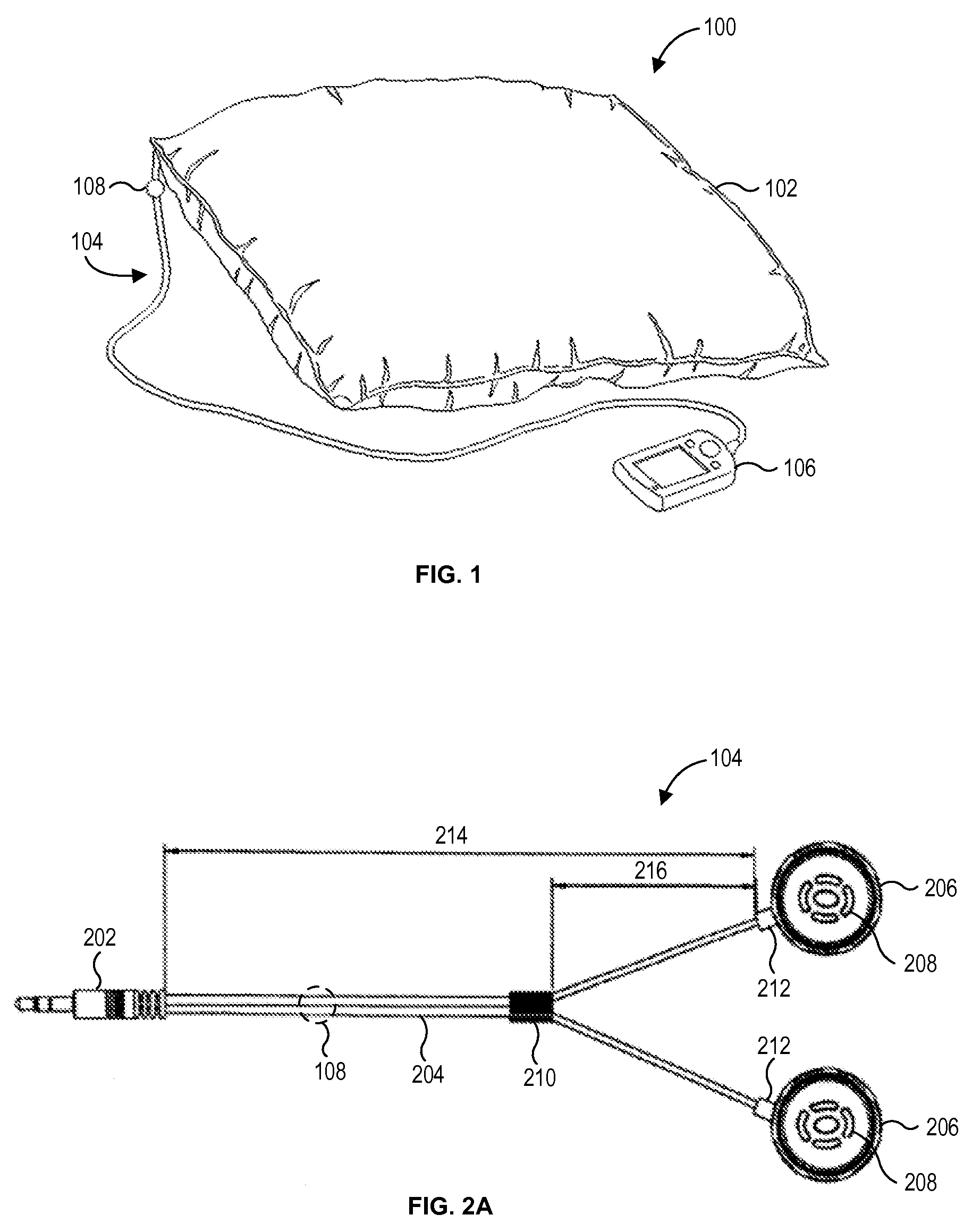
Sound pillow sleep system
Provided is a pillow and method of marking the pillow to mitigate tinnitus. The pillow includes a first and second portions of fill material, an anchor strip assembly and a pillow casing. The anchor strip assembly is disposed between the first and second portions of fill material. The anchor strip assembly includes an anchor strip and a plurality of speakers secured to the anchor strip at a distance from each other. A first and second edge portions the anchor strip extends beyond edges of the first and second portions of fill material. The pillow casing secures the anchor strip assembly disposed between the first and second portions of the fill material inside the pillow casing such that the first and second edge portions of the anchor strip are secured respectively between a first and second seams of the pillow casing.
Owner:ARMBRUSTER ENTERPRISES
Delivery of modulators of glutamate-mediated neurotransmission to the inner ear
The invention features methods and devices for local delivery of agents that modify glutamate-mediated neurotransmission to the inner ear for treatment of inner ear disorders caused by glutamate-induced hearing loss and / or tinnitus.
Owner:DURECT CORP
High fidelity hearing restoration
InactiveUS20050013445A1Accurately measuring hearing lossElectrotherapyAudiometeringRestoration methodElectrophonic hearing
Method for accurately measuring hearing loss includes the steps of selecting a series of audio tones within the normal range of hearing (502) and then measuring a relative sensitivity of a test subject with respect to the ability to hear each of the audio tones, exclusive of the effects of tinnitus. (504, 506, 508, 510, 512) The relative sensitivity of the test subject to hear the tones can be measured by determining (510) for each tone an intensity necessary for the test subject to hear the tones at a subjectively equal loudness level which is selected to exceed a perceived level of noise attributable to tinnitus for the test subject.
Owner:TECH LICENSING CORP (US)
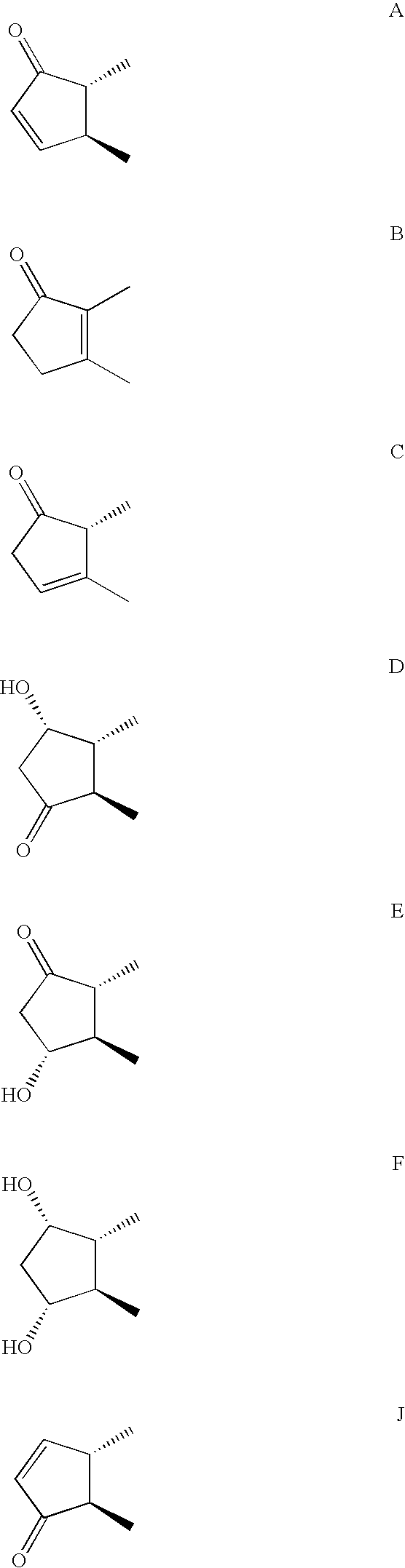
2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones
The present invention provides novel 2-pyridinyl[7(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-3-yl]methanones with at least one substituent on both the 2- and 4-pyridinyl ring having the chemical structure of formula I: The invention further provides compositions and methods employing the novel 2-pyridinyl[7-(pyridin-4-yl)pyrazolo[1,5-a]pyrimidin-3-yl]methanones of formula I to modulate GABA and GABAA receptor physiology to elicit therapeutic responses in mammalian subjects to alleviate neurological or psychiatric disorders, including stroke, head trauma, epilepsy, pain, migraine, mood disorders, anxiety, post traumatic stress disorder, obsessive compulsive disorders, mania, bipolar disorders, schizophrenia, seizures, convulsions, tinnitus, neurodegenerative disorders including Alzheimer's disease, amyotrophic lateral sclerosis and Parkinson's disease, Huntington's chorea, depression, bipolar disorders, mania, trigeminal and other neuralgia, neuropathic pain, hypertension, cerebral ischemia, cardiac arrhythmia, myotonia, substance abuse, myoclonus, essential tremor, dyskinesia and other movement disorders, neonatal cerebral hemorrhage, and spasticity, as well as other psychiatric and neurological disorders mediated by GABA and / or GABAA receptors.
Owner:SKOLNICK PHIL +1
Soft, middle-ear electrode for suppressing tinnitis
InactiveUS20070213787A1Easy to insertSuppressing tinnitusElectric tinnitus maskersImplantable neurostimulatorsMedicineMiddle ear
A soft, ball-shaped middle-ear electrode is inserted and wedged into the natural cavity that exists in front of the round window. An electrical pulse generator connected to the soft, ball-shaped electrode provides electrical stimulation to the region surrounding the round window for the purpose of suppressing tinnitus or to improve hearing.
Owner:BOSTON SCI NEUROMODULATION CORP
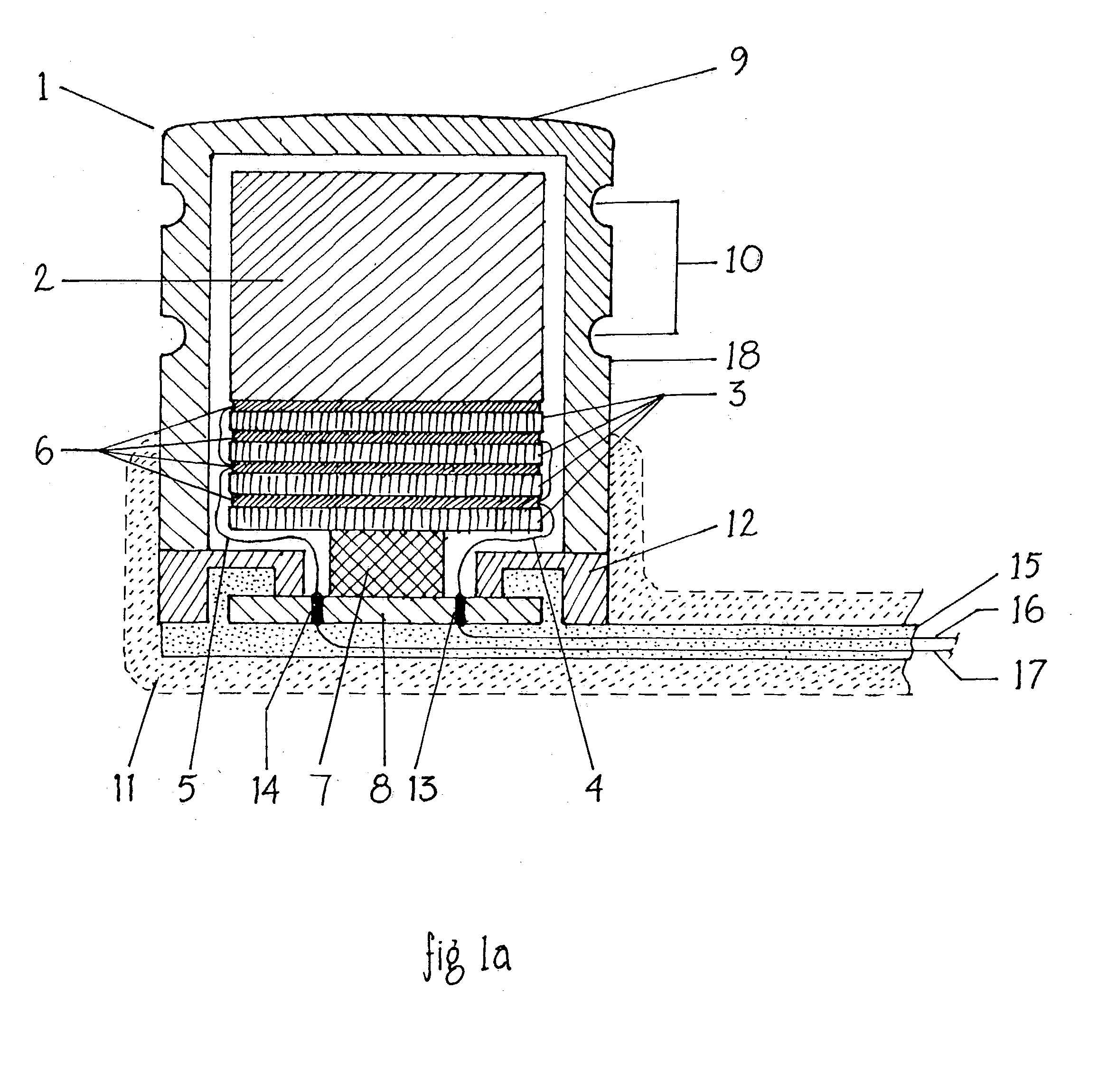
Endosteal electrode
InactiveUS20070282416A1Improve hearingMaximises possibilityHead electrodesEar treatmentCochlear implantationEngineering
An implantable tissue-stimulating device comprising an elongate electrode carrier member (11) having a plurality of electrodes thereon. The electrodes are preferably disposed in a linear array on the carrier member (11) and are adapted to apply a preselected tissue stimulation to the cochlea. The carrier member (11) is preformed from a resiliently flexible biocompatible silicone and extends from a distal end (12) to a stop member (13). The carrier member (11) is adapted for intracochlear but extraluminar insertion within the cochlea of an implantee. In particular, the carrier member (11) is adapted to be implanted in the crevice (21) between the spiral ligament (22) and the endosteum (23) of the lateral wall of the cochlea (20). This is a quite different location to the normal placement of the cochlear implant electrode array in the scala tympani (24) of the cochlea (20). The placement of the carrier member (11) is designed to avoid any breach of the internal ducts of the cochlea (20), such as the scala tympani (24) and scala vestibuli (25) so that the normal hydrodynamic behaviour of the cochlea (20) is not affected by any intrusive device. By preservng the normal hydrodynamic behaviour of the cochlea (20), use of the carrier member (11) maximises the possibility of also preserving any hearing of the implantee that is offered by the cochlea (20). Use of the device in a system for masking or treating the symptoms of tinnitus is also described.
Owner:COCHLEAR LIMITED
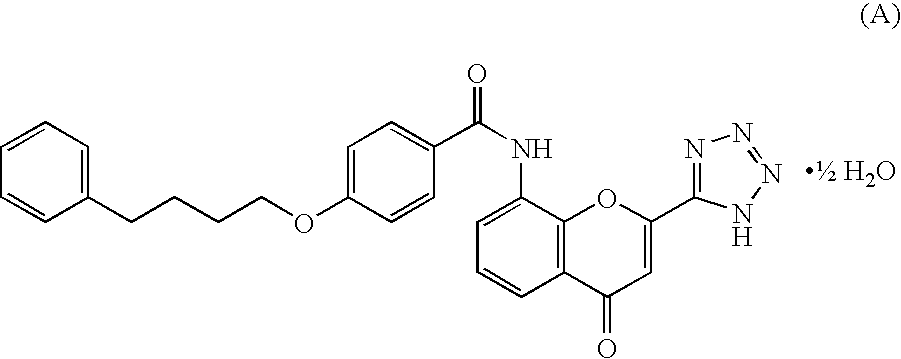
Ambroxol for the treatment of tinnitus
A method of treating an otological disorder in a patient in need thereof, the method comprising administering to the patient ambroxol or a pharmacologically acceptable salt thereof.
Owner:BOEHRINGER INGELHEIM INT GMBH
Traditional Chinese medicine decoction piece combination preparation enhancing body immunity, preparation method therefor and combination package
The invention relates to a traditional Chinese medicine decoction piece preparation, and concretely relates to a traditional Chinese medicine decoction piece combination preparation enhancing body immunity, a preparation method therefor and combination package. The raw materials of the traditional Chinese medicine decoction piece combination preparation are composed of radix ophiopogonis, Chinese magnoliavine, prepared rehmannia root, cornus officinalis, tree peony bark, Chinese yam, poria cocos and oriental waterplantain rhizome. The traditional Chinese medicine decoction piece combination preparation has effects of nourishing kidney and lung, is used for treating lung and kidney yin deficiency, tidal fever, night sweating, pharynx dryness, dizziness, tinnitus and soreness of loins and knees, can enhance body immunity and has functions of anti-fatigue and conditioning of the sub-health state.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
System and method for ear-arranged transcutaneous vagus nerve stimulation
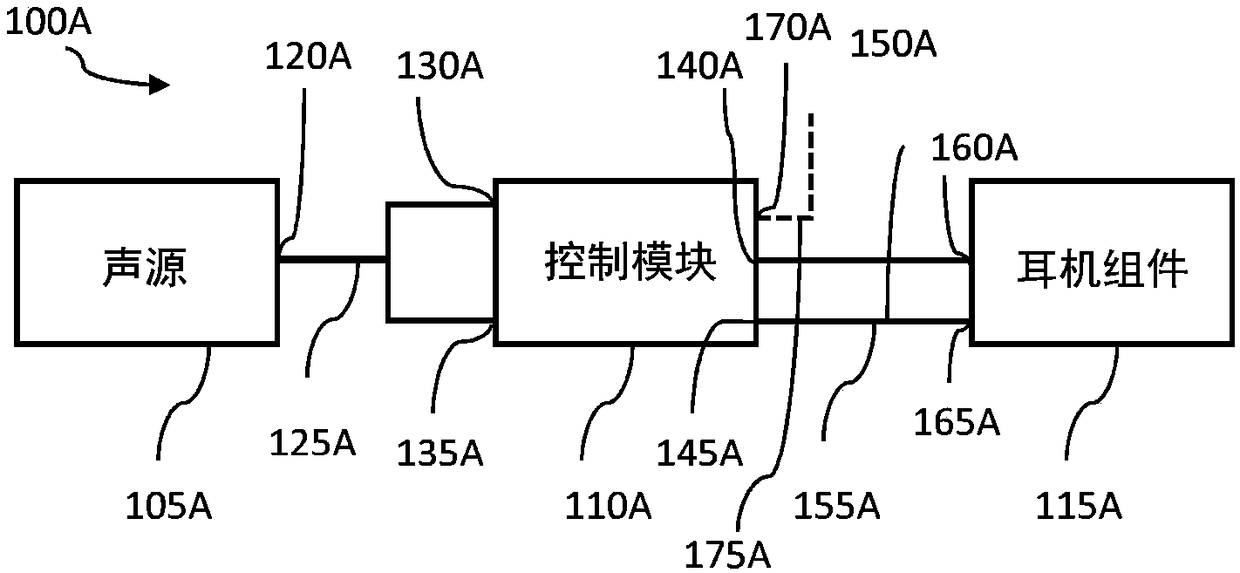
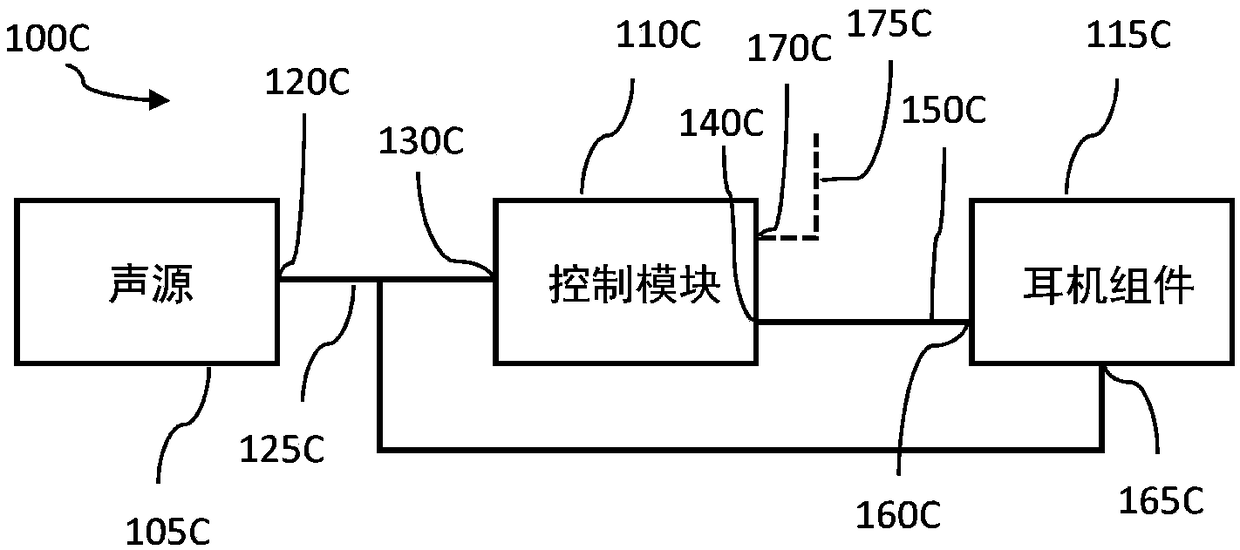
InactiveCN109069297AConvenient treatmentImprove convenienceElectrotherapyMedical devicesSound sourcesEngineering
A transcutaneous nerve stimulation system includes a multifunctional earphone assembly that receives a sound signal from a sound source or other sources (such as a signal generator). An amplifier receiving the sound signal from the sound source or independent signal generator amplifies the sound signal to generate an amplified electrical signal. A stimulator, which includes multiple conductive electrode contacts, is coupled to the amplifier to receive the amplified electrical signal. The electrode contacts may protrude from an earbud / headphone or extend from an adjustable probe arm. The amplified electrical signal is used to apply electrical stimulation while the earphone assembly emits audible sounds according to the sound signal. The combined functionality enhances compliance with treatment regimens involving electrical stimulation of vagus nerves and other regions of a subject. Potential indications include, without limitation, major depressive disorder, epilepsy, chronic pain, pre-diabetes, insomnia, cardiovascular disorders, tinnitus, autism, daily stress, and anxiety.
Owner:THE GENERAL HOSPITAL CORP
Health-care red wine and preparation method thereof
InactiveCN104109596ARelieve dizzinessRelieve tinnitusDigestive systemWine preparationIntestinal structureCervical spondylosis
The invention discloses a health-care red wine and a preparation method thereof. The red wine base of the health-care red wine also comprises the following raw materials: 0.5-1.5 g / L raspberry, 0.01-1.5 g / L Rhodiola rosea, 0.01-1.5 g / L dodder, 0.01-1.5 g / L radix rehmanniae, 0.1-1 g / L Chinese wolfberry, 0.1-1 g / L mulberry, 0.05-0.8 g / L radix astragali, 0.05-0.8 g / L tuckahoe and 0.01-0.8 g / L licorice. The health-care red wine is prepared from multiple medicinal materials, has the functions of consolidating the constitution, benefiting the intestine, tonifying the kidney, strengthening the spleen, regulating sleep, warming the stomach, resisting aging and softening the blood vessels, has obvious effects on regulating frequent urination, precipitant urination, insomnia and discomfort caused by heart and cerebral vessels, and can relieve dizziness, tinnitus, nausea and other symptoms caused by cervical spondylosis.
Owner:徐昕然
Preparation method of health liquor
ActiveCN104893897AIncrease profitGood health effectHeavy metal active ingredientsAnthropod material medical ingredientsMedicinal herbsPalpitations
The invention discloses a preparation method of a health liquor. The preparation method comprises the following steps: step 1, making base liquor of the health liquor; step 2, making the health liquor by matching the base liquor and medicinal materials; step 3, sealing and storing; and step 4, bottling and packaging. In the processes of liquor brewing, medicinal material soaking and blending, medicinal properties can be completely released into the base liquor after the medicinal materials are soaked into the base liquor and therefore the utilization rate of the medicinal materials is high; the health liquor has outstanding treatment effects on poor sleep, gastrointestinal discomfort, aching lumbus and limp legs, kidney-deficiency impotence, physical and mental fatigue, tinnitus and heart palpitation, has the functions of worming and smoothening blood vessels, strengthening heart and tonifying yang and thus has good health care effect.
Owner:邓杨 +1
Preventive and/or Therapeutic Agents for Meniere's Disease
InactiveUS20070249695A1Effective preventionEffective therapeutic agentBiocideSenses disorderInner Ear DiseasesMedicine
The present invention relates to preventive and / or therapeutic agents for Meniere's disease, which comprise a leukotriene antagonist (such as pranlukast hydrate) as an active ingredient. Leukotriene antagonists (such as pranlukast hydrate) are effective in ameliorating various symptoms, such as hearing impairment, tinnitus, a feeling of fullness in the ear and vertigo, thus being useful as a preventive and / or therapeutic agent for Meniere's disease.
Owner:ONO PHARMA CO LTD +1
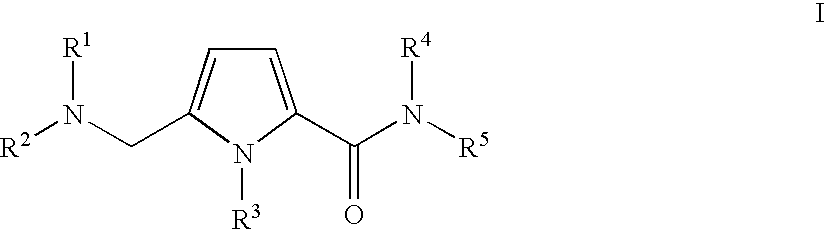
Substituted 5-aminomethyl-1H-pyrrole-2-carboxylic acid amides
Substituted 5-aminomethyl-1H-pyrrole-2-carboxylic acid amides, a process for the production thereof, pharmaceutical preparations containing these compounds and the use of these compounds in pharmaceutical preparations for treatment or inhibition of withdrawal symptoms, memory disorders, neurodegenerative diseases, epilepsy, cardiovascular disorders, water retention, intestinal motility disorders, urinary incontinence, anorexia, tinnitus, pruritus, depression, sexual dysfunction, airways diseases, food intake disorders, or type II (non-insulin-dependent) diabetes, or for anxiolysis, diuresis, suppression of the urinary reflex, reducing the addictive potential of opioids, modulating locomotor activity, influencing the cardiovascular system, or regulating electrolyte balance.
Owner:GRUNENTHAL GMBH
Noise damage tinnitus treating device
InactiveCN1507841ASimple structureEasy to useElectrotherapyEar treatmentElectrical resistance and conductanceEngineering
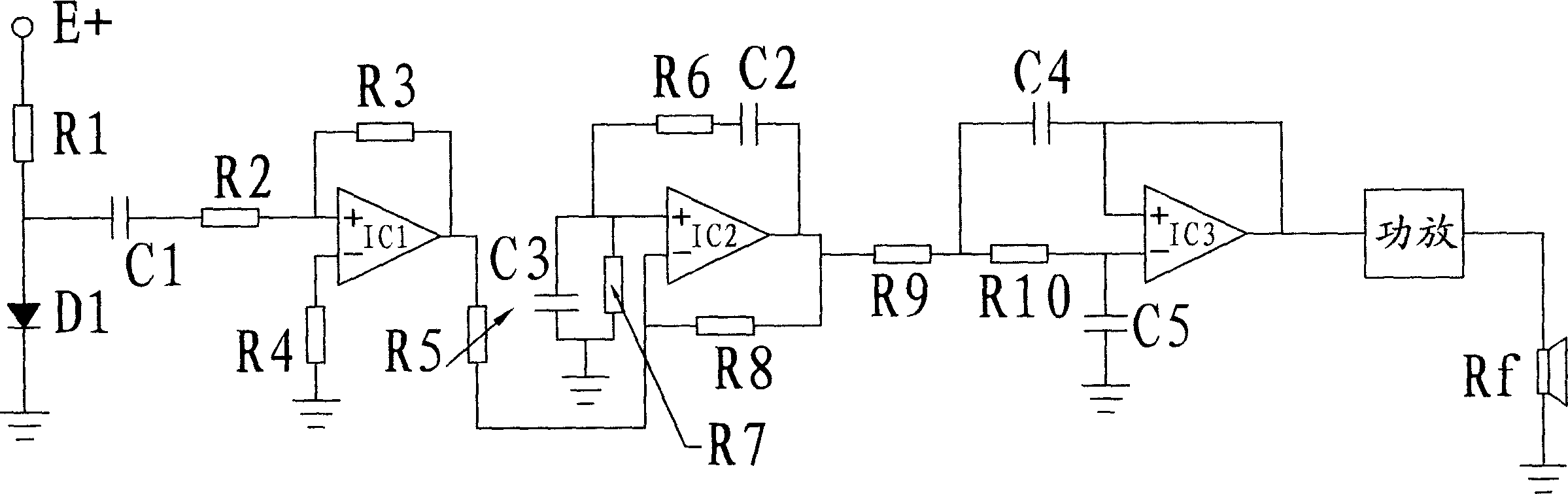
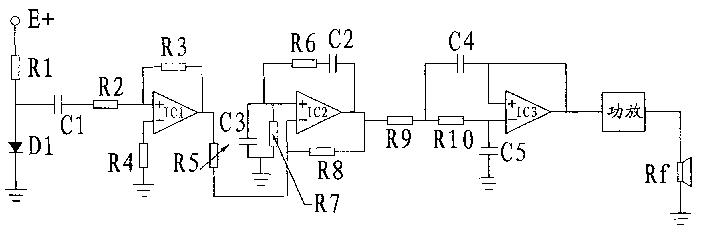
The present invention relates to a therapeutic equipment for curing tinnitus due to noise hazard. The connection relationship between all the composition circuits is noise source circuit, it is connected with one end of resistor R2 in amplification circuit by means of capacitor C1, the output end of amplification circuit is connected with one end of resistor R5 is active narrow-band filter, and the output end of the active narrow-band filter is connected with one end of resistor R9 of high pass active filter, and the output end of high pass active filter is connected with input end of power amplification circuit, and the output end of power amplification circuit is connected with earphone.
Owner:深圳市丰河环境工程技术有限公司上海分公司
Novel method and composition for local treatment of meniere's disease, tinnitus and/or hearing loss
Novel methods and compositions for the local treatment of Meniere's disease, tinnitus and hearing loss employ a therapeutically effective amount of a prostaglandin of the F-type to the inner ear. The treatment can be either continuous or intermittent and may involve the use of pumps, gels, or slow release drug inserts.
Owner:SYNFOLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

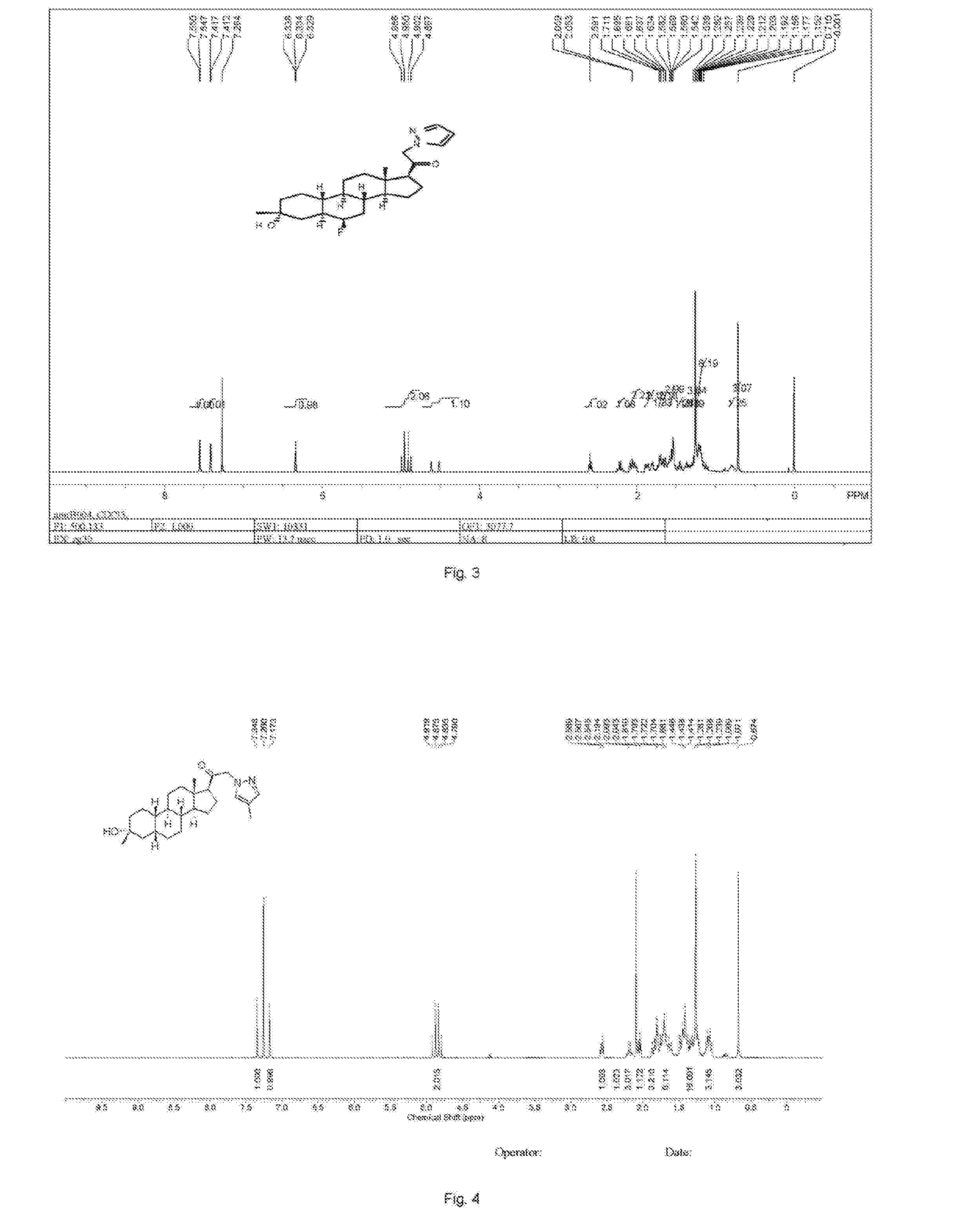
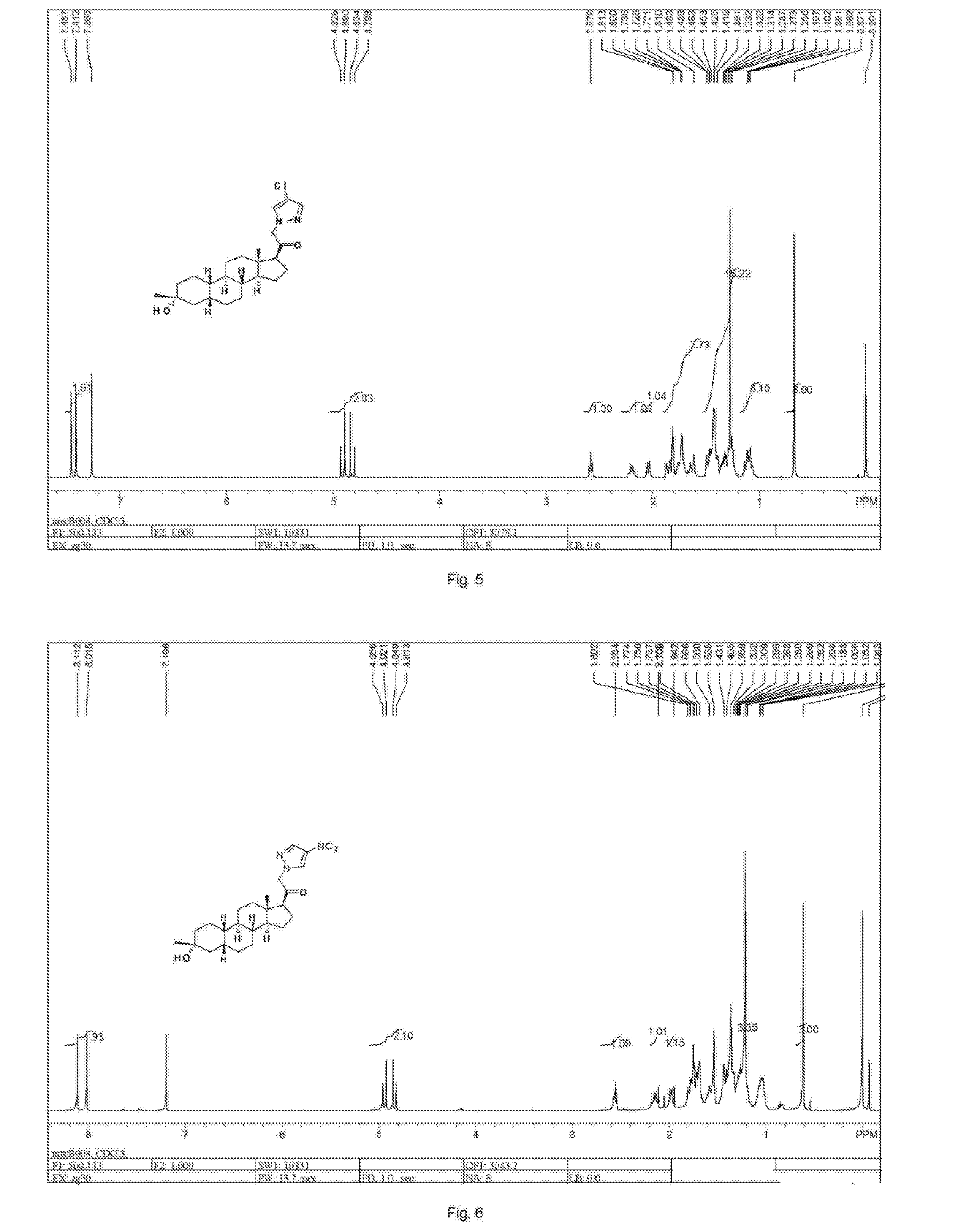
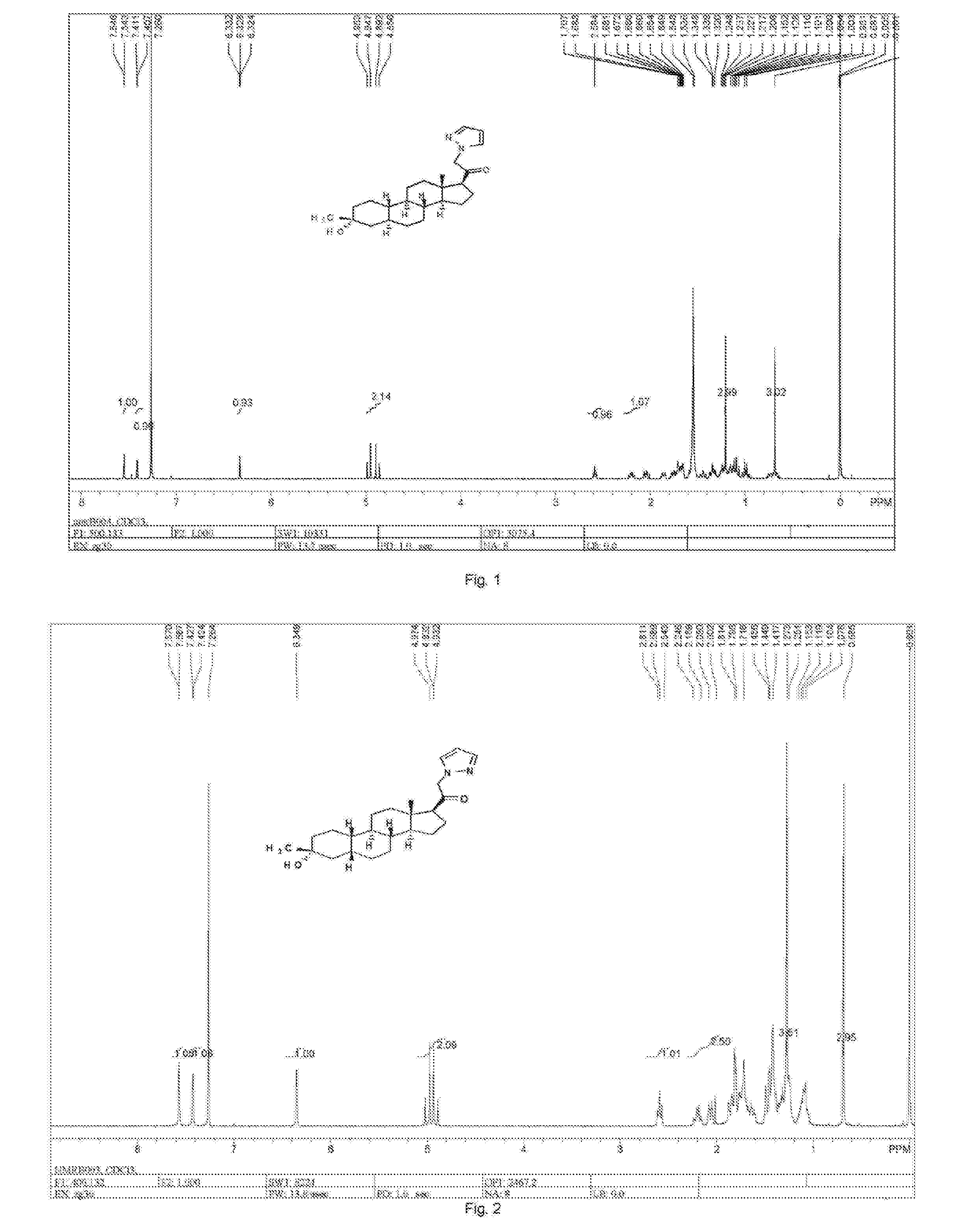
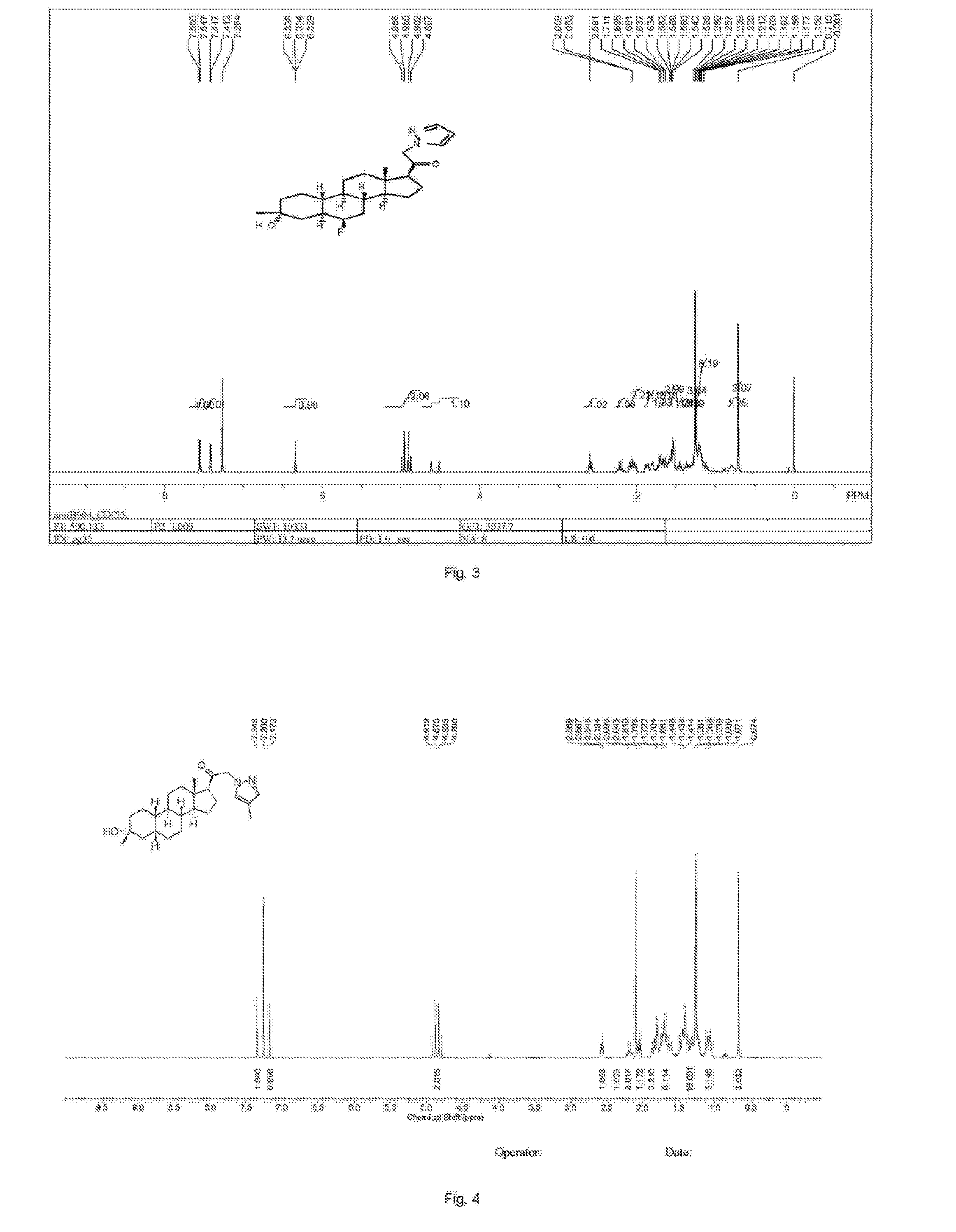
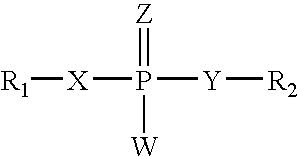






![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00001.png)
![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00002.png)
![2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones 2-pyridinyl[7-(substituted-pyridin-4-yl) pyrazolo[1,5-a]pyrimidin-3-yl]methanones](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d5295254-0381-4a85-b180-3ea833a7a1eb/US20050245517A1-20051103-C00003.png)