Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
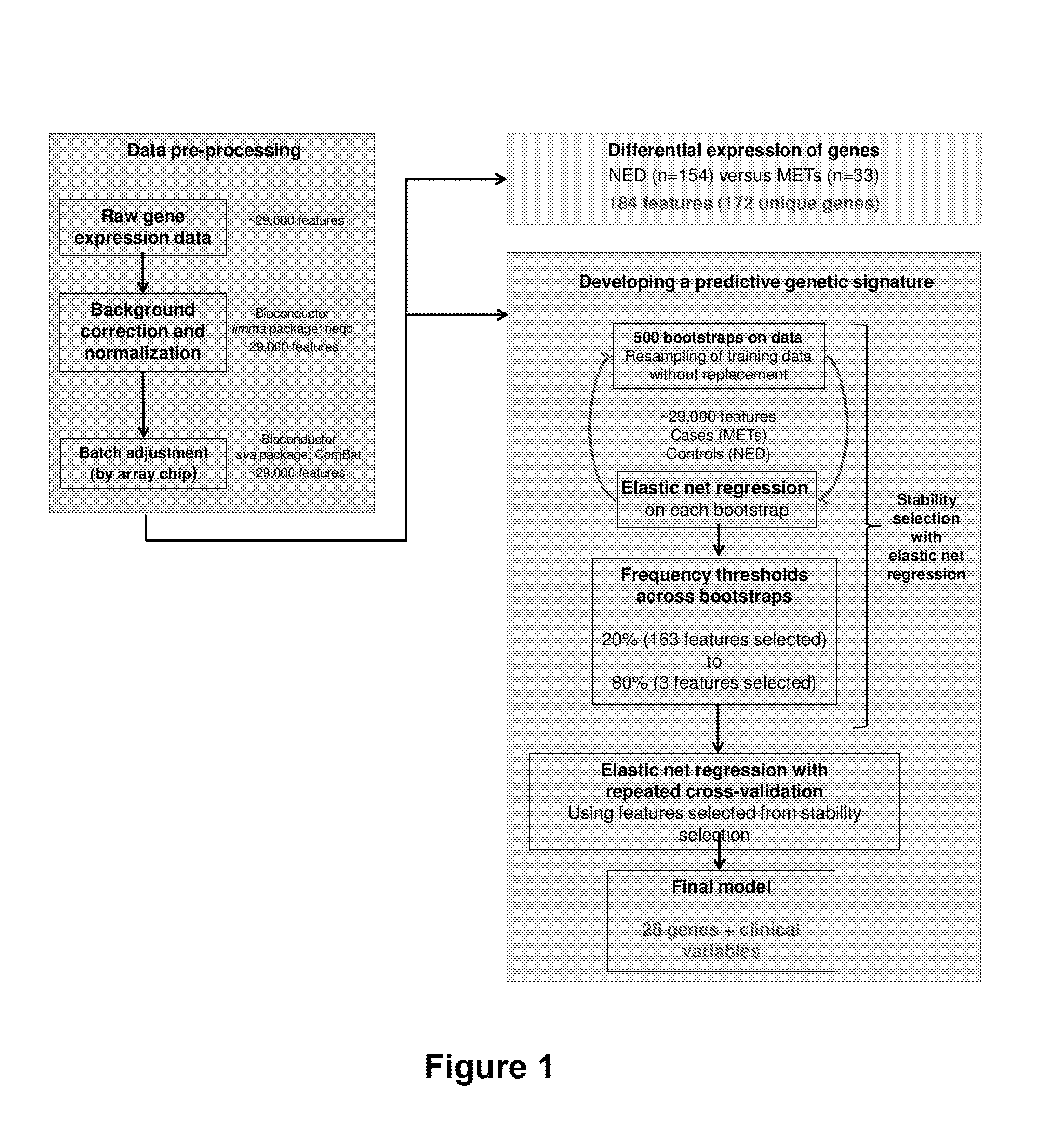
41 results about "PCA3" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
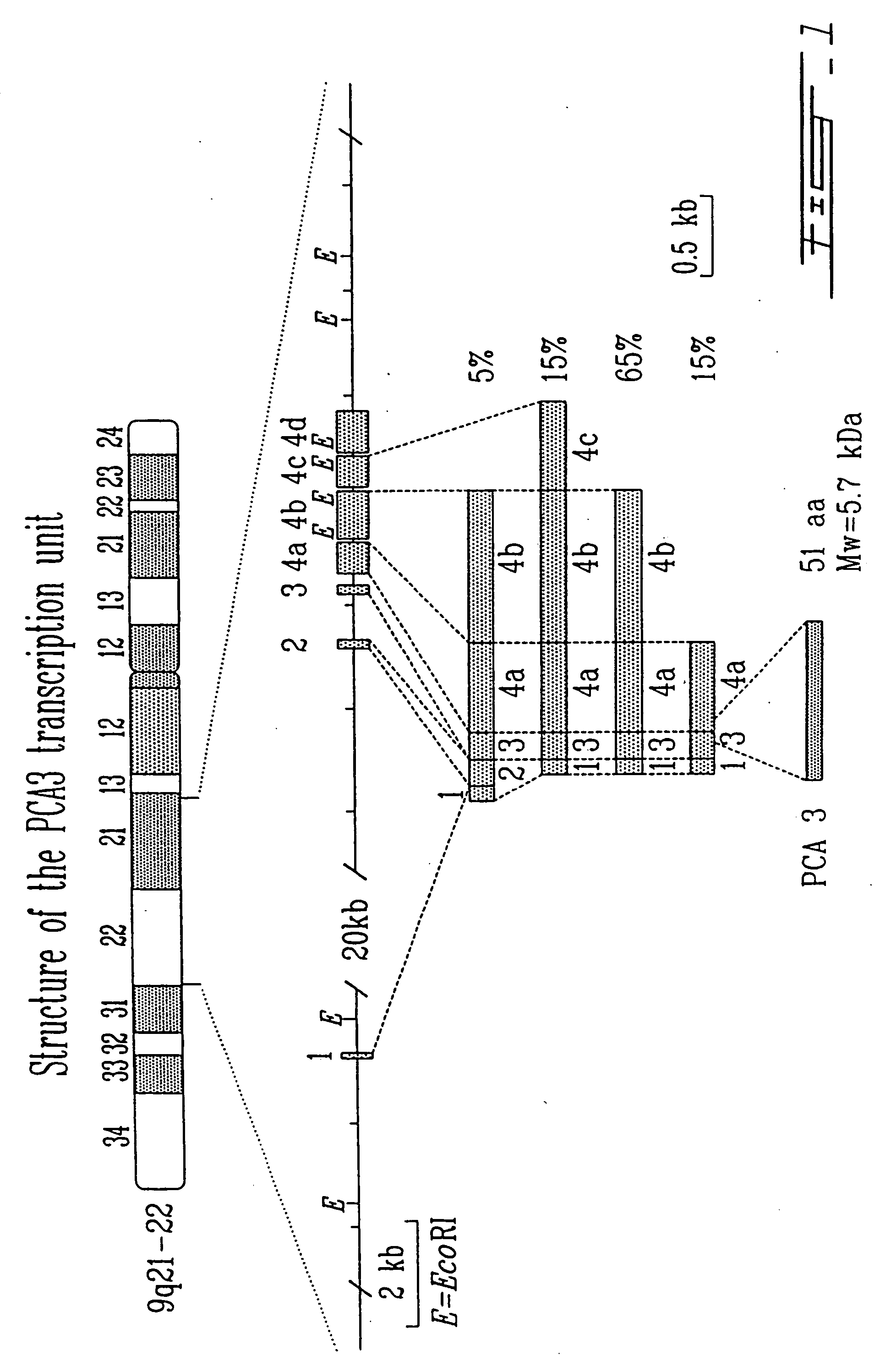
Prostate cancer antigen 3 (PCA3, also referred to as DD3) is a gene that expresses a non-coding RNA. PCA3 is only expressed in human prostate tissue, and the gene is highly overexpressed in prostate cancer. Because of its restricted expression profile, the PCA3 RNA is useful as a tumor marker.
PCA3, PCA3 genes, and methods of use
InactiveUS7008765B1Promote absorptionImprove biological half lifeOrganic active ingredientsBacteriaAntigenNucleic Acid Probes
The present invention relates, in general, to a prostate-specific antigen, PCA3. In particular, the present invention relates to nucleic acid molecules coding for the PCA3 protein; purified PCA3 proteins and polypeptides; recombinant nucleic acid molecules; cells containing the recombinant nucleic acid molecules; antibodies having binding affinity specifically to PCA3 proteins and polypeptides; hybridomas containing the antibodies; nucleic acid probes for the detection of nucleic acids encoding PCA3 proteins; a method of detecting nucleic acids encoding PCA3 proteins or polypeptides in a sample; kits containing nucleic acid probes or antibodies; bioassays using the nucleic acid sequence, protein or antibodies of this invention to diagnose, assess, or prognose a mammal afflicted with prostate cancer; therapeutic uses; and methods of preventing prostate cancer in an animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Nucleic acid molecules comprising the promoter for PCA3, and uses thereof
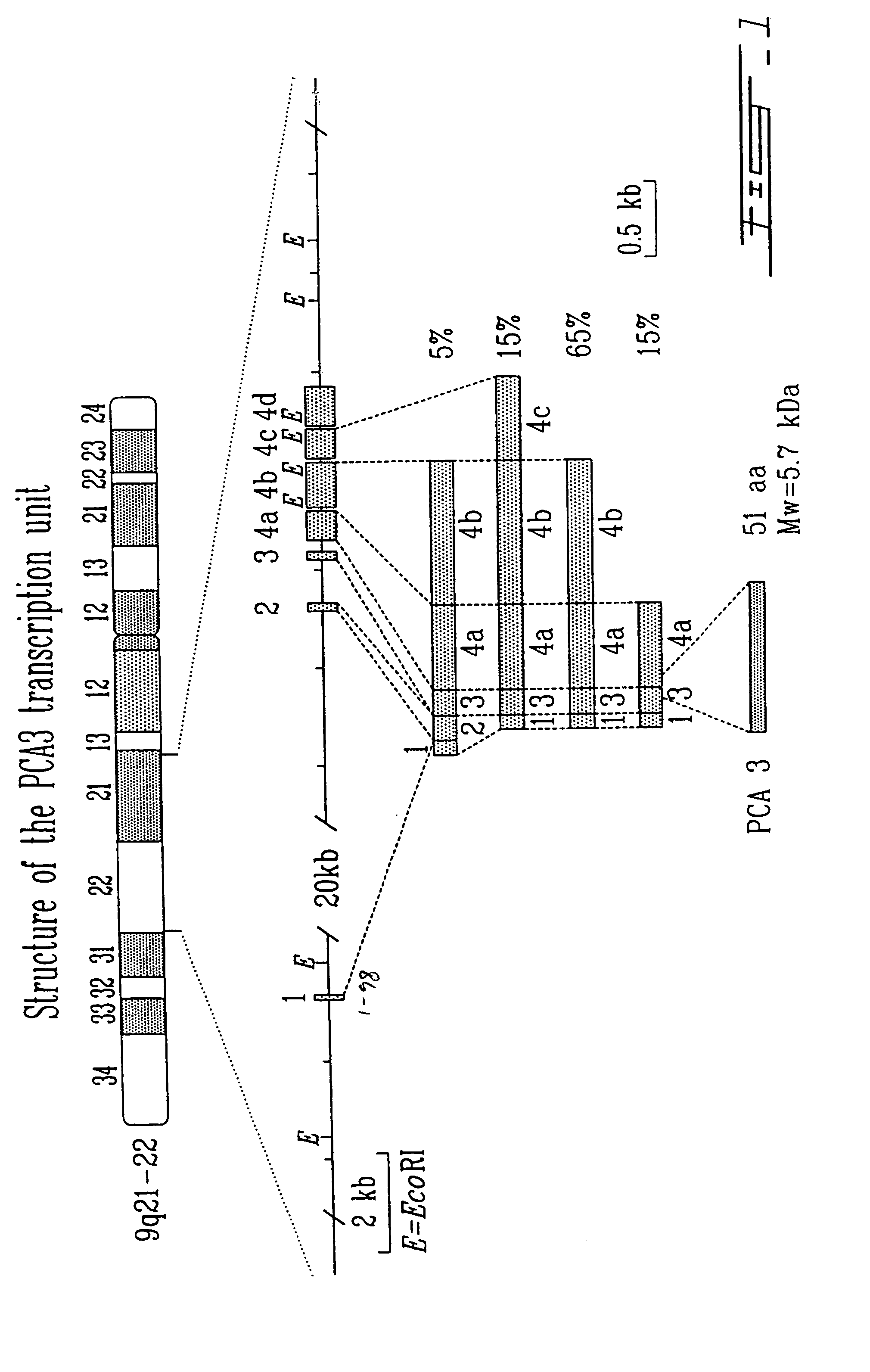
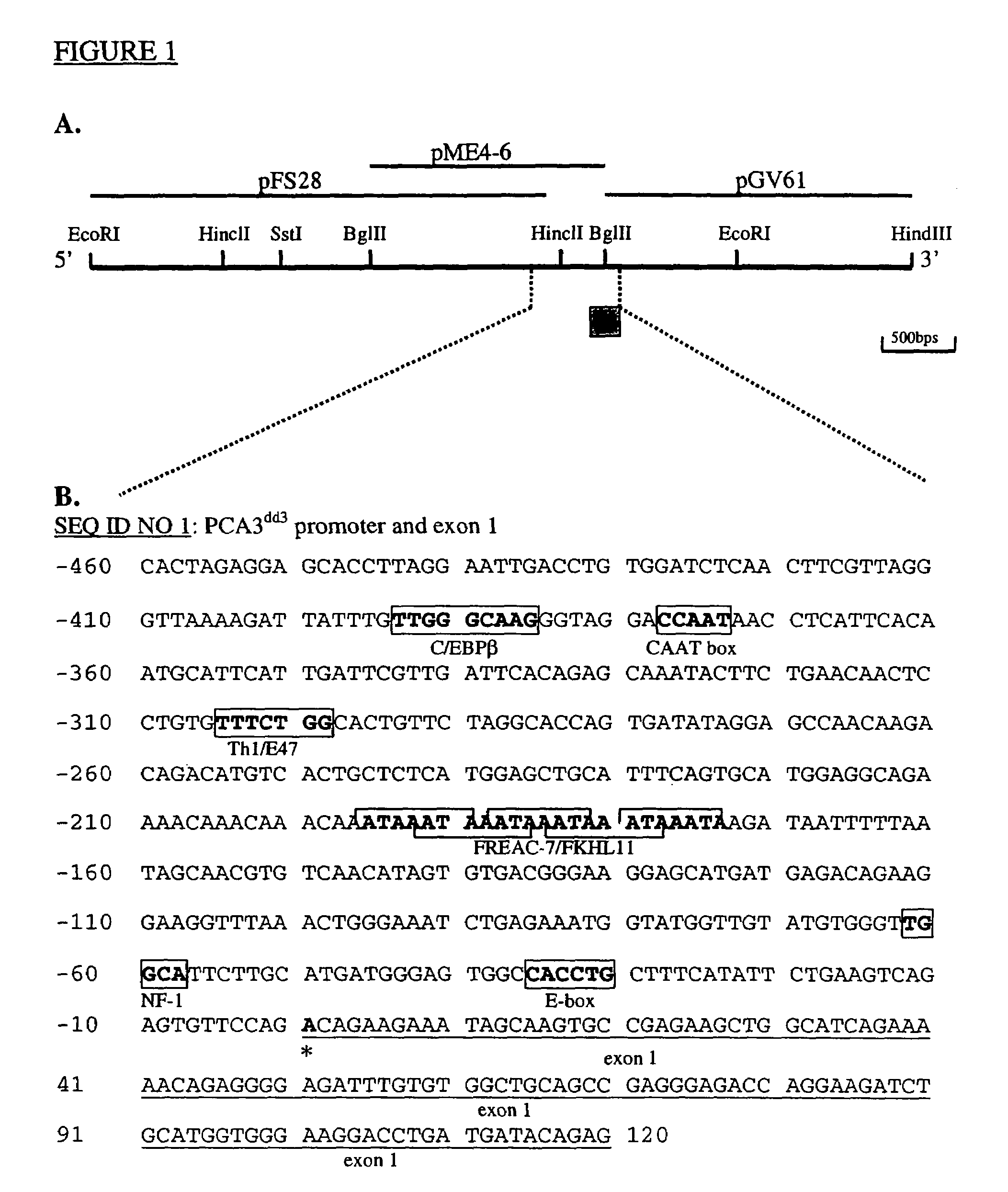
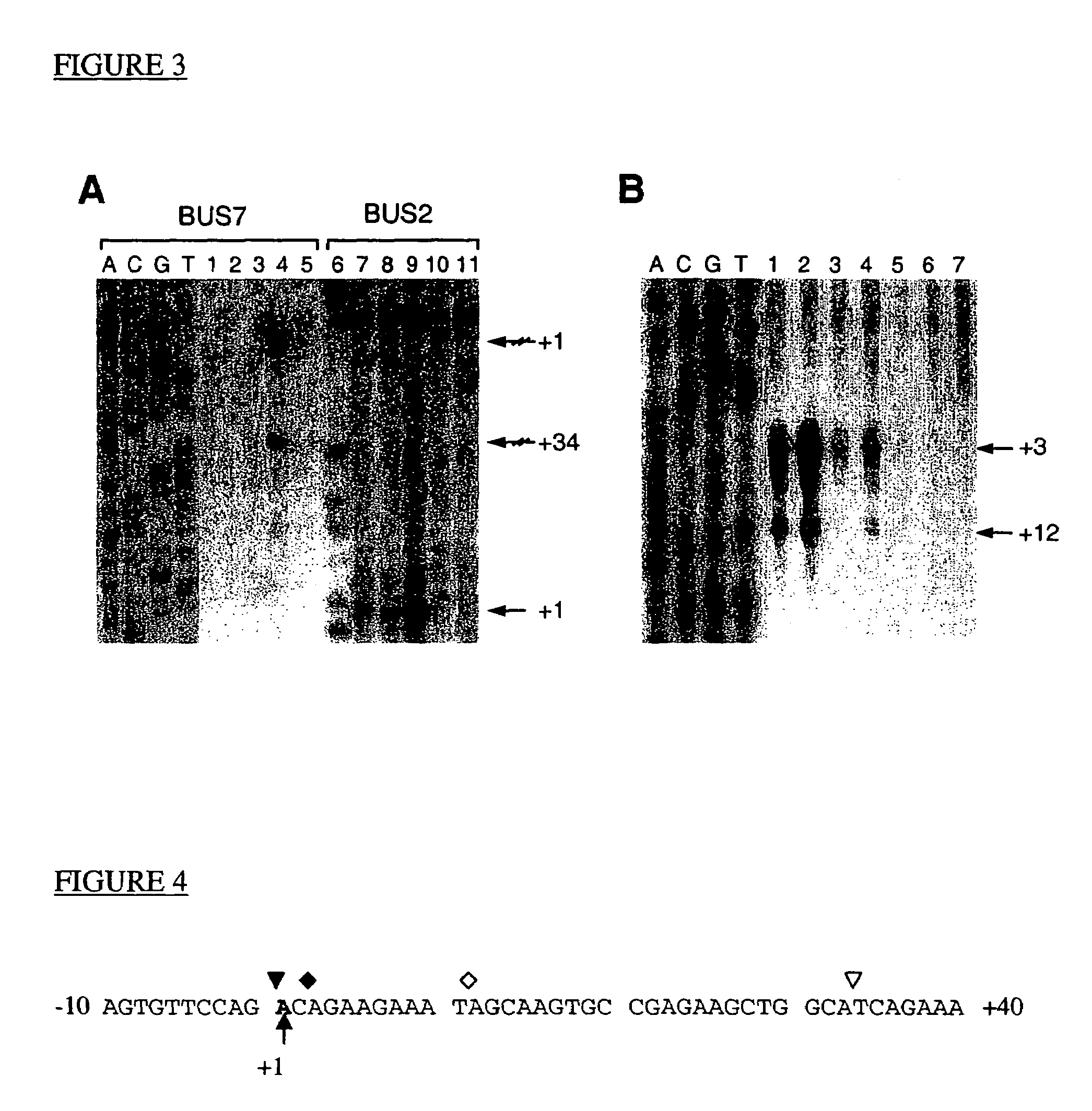
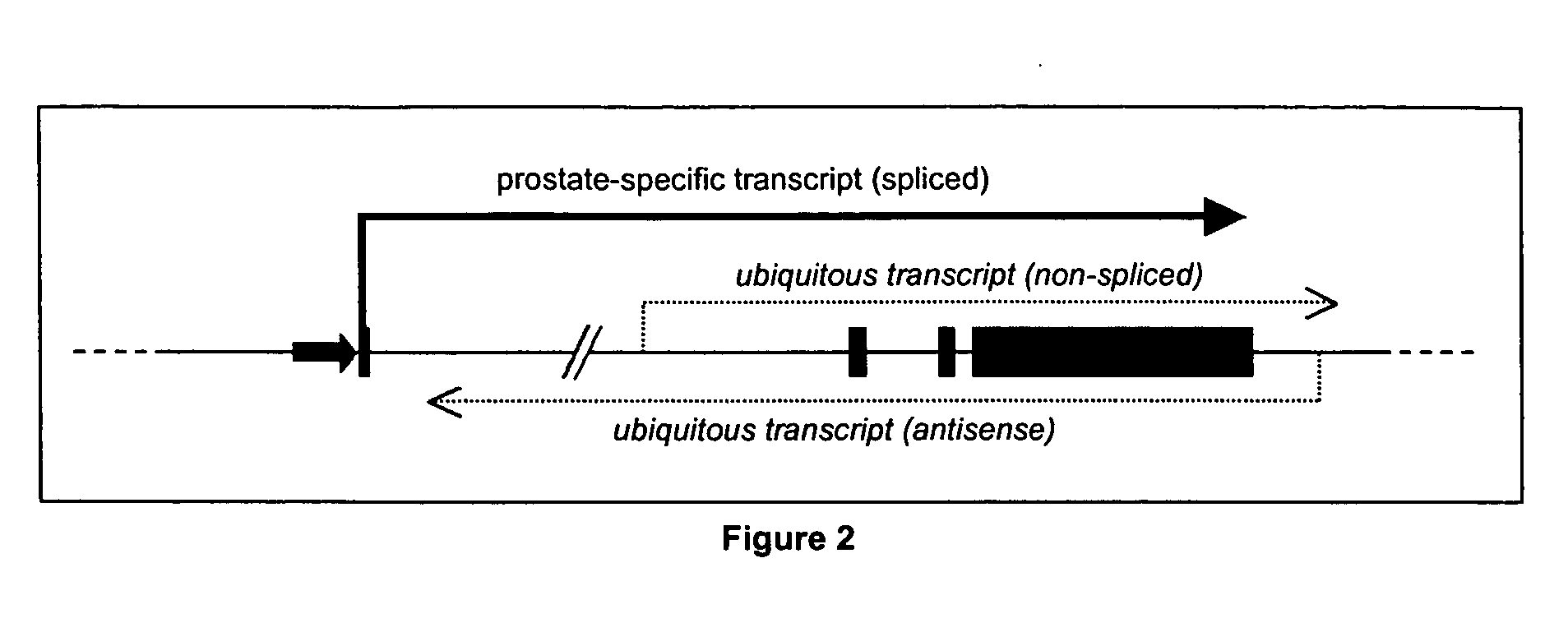
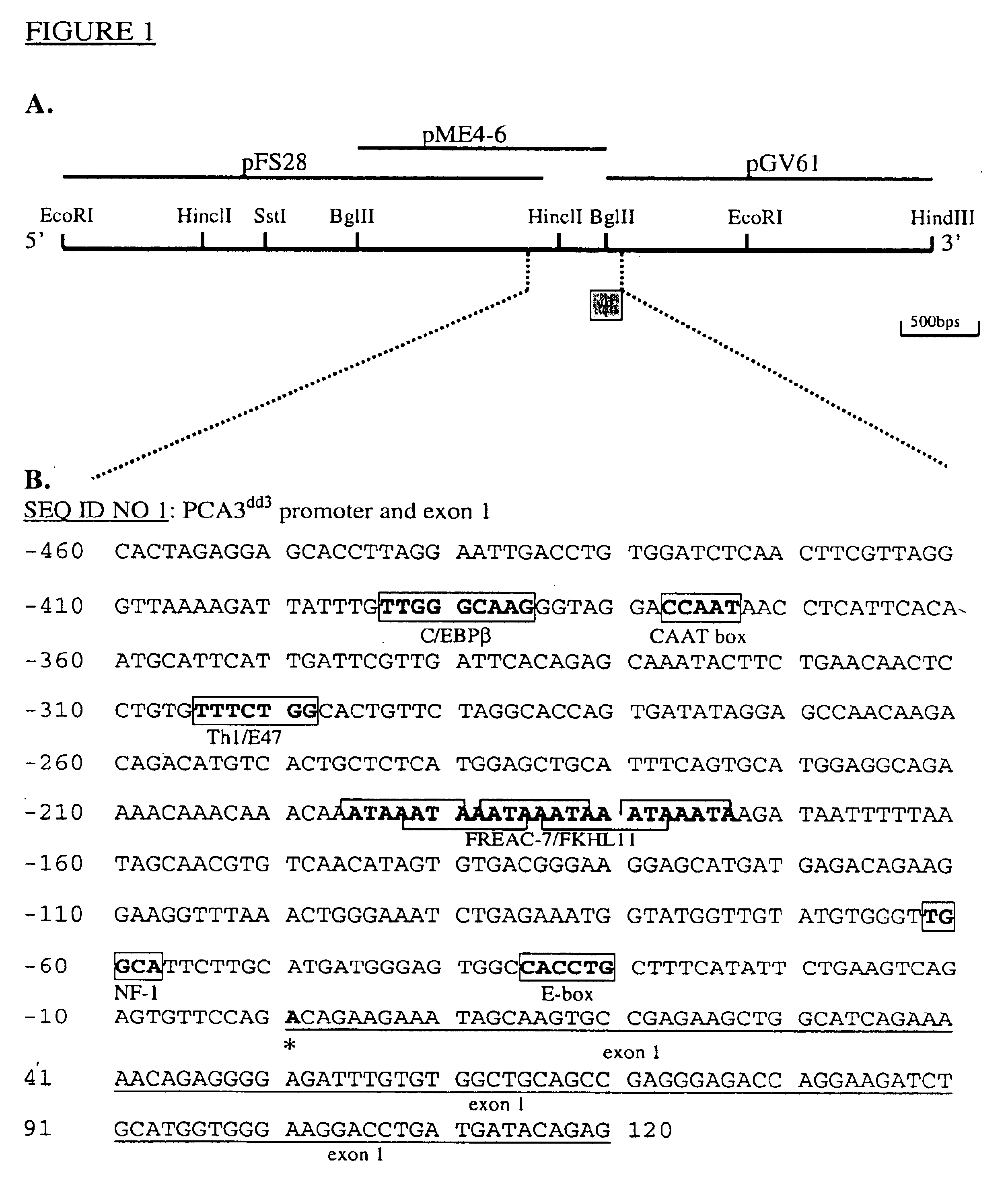
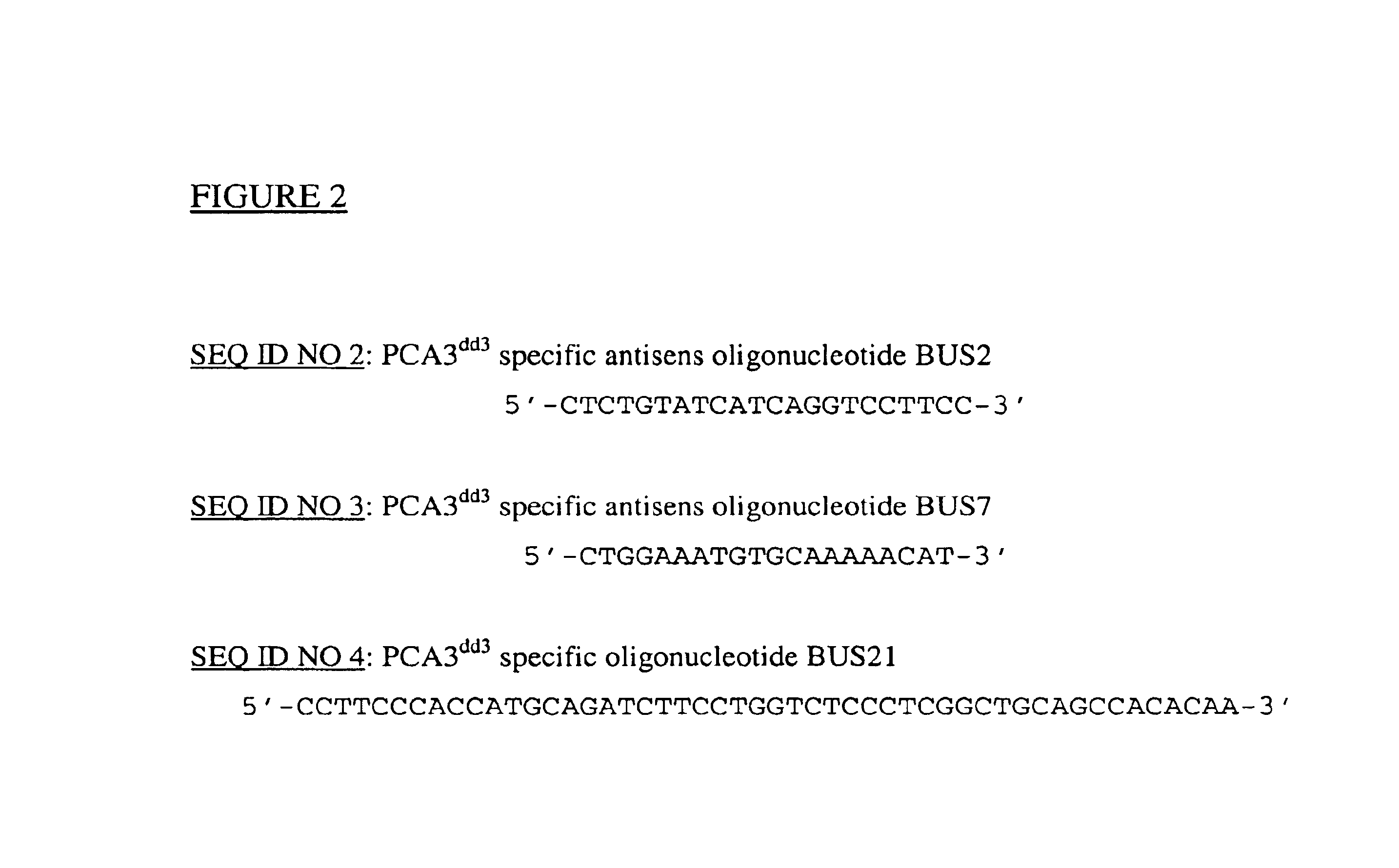
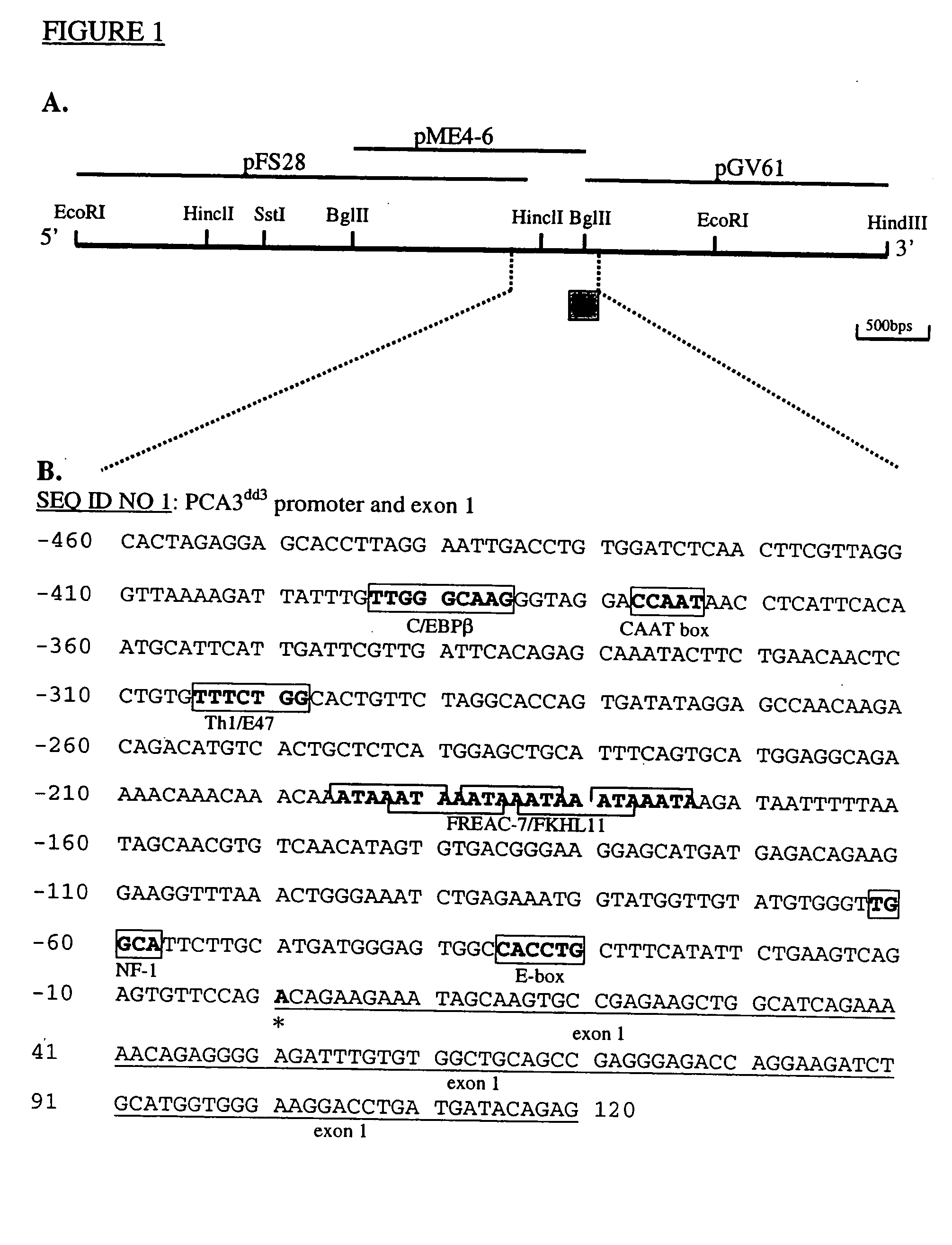
This invention describes the cloning and characterization of the promoter region for the PCA3dd3 gene. This region regulates the PCA3dd3 gene expression by a unique prostate specific transcriptional mechanism. The present invention relates to the use of this promoter region as a tool for prostate cancer treatment and diagnosis and screening of agents which regulate expression of PCA3. In a particular embodiment, the present invention relates to an isolated promoter sequence which comprises an isolated promoter sequence which comprises a sequence as set forth between nucleotide positions 372 to 460 of SEQ ID NO:1, this sequence enabling a prostate-specific modulation of transcription.
Owner:STICHTING KATHOLIEKE UNIV
Specific method of prostate cancer detection based on PCA3 gene and kits therefor
InactiveUS20050164223A1Strong specificityHigh detection sensitivityMicrobiological testing/measurementNucleic Acid ProbesExon
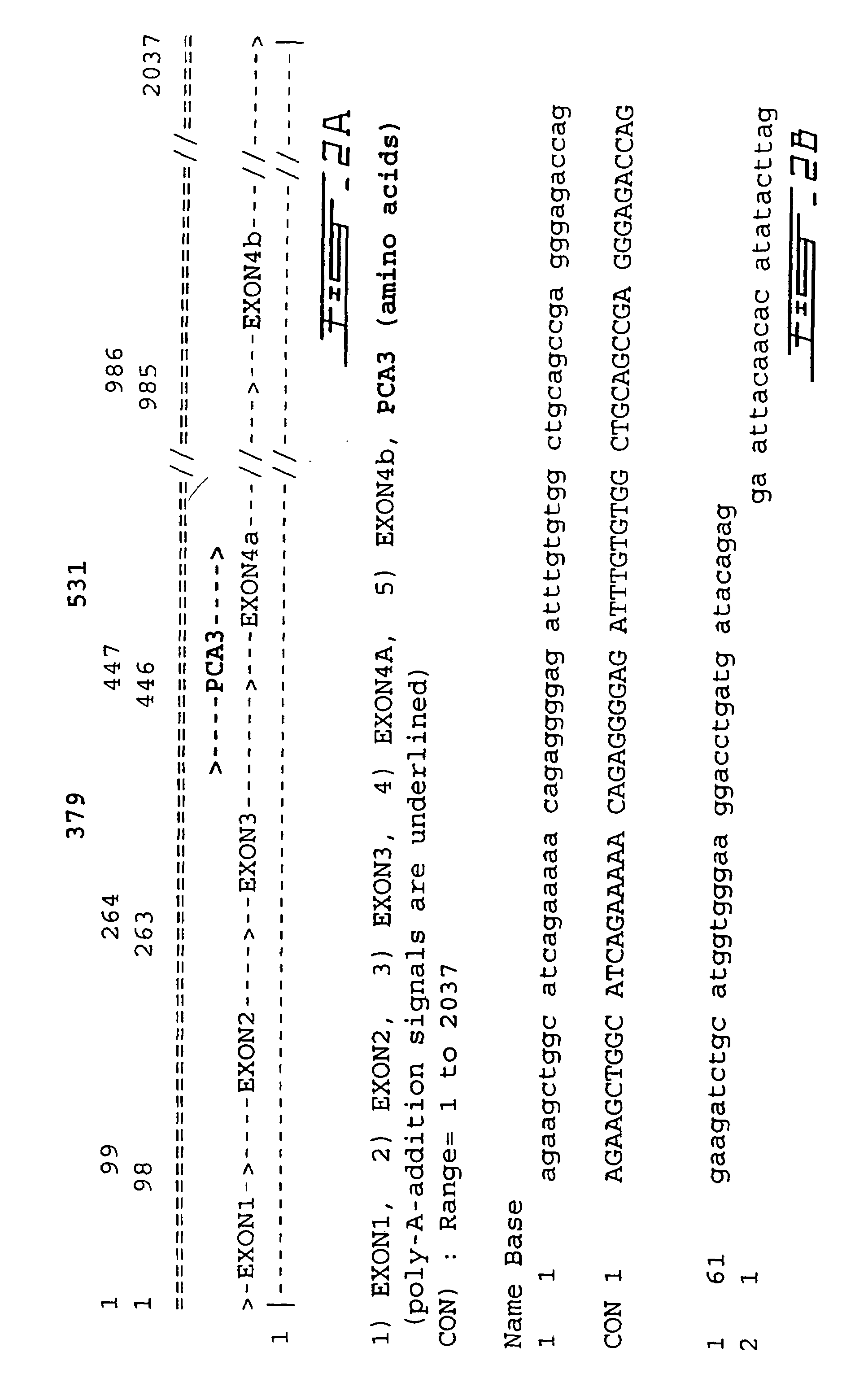
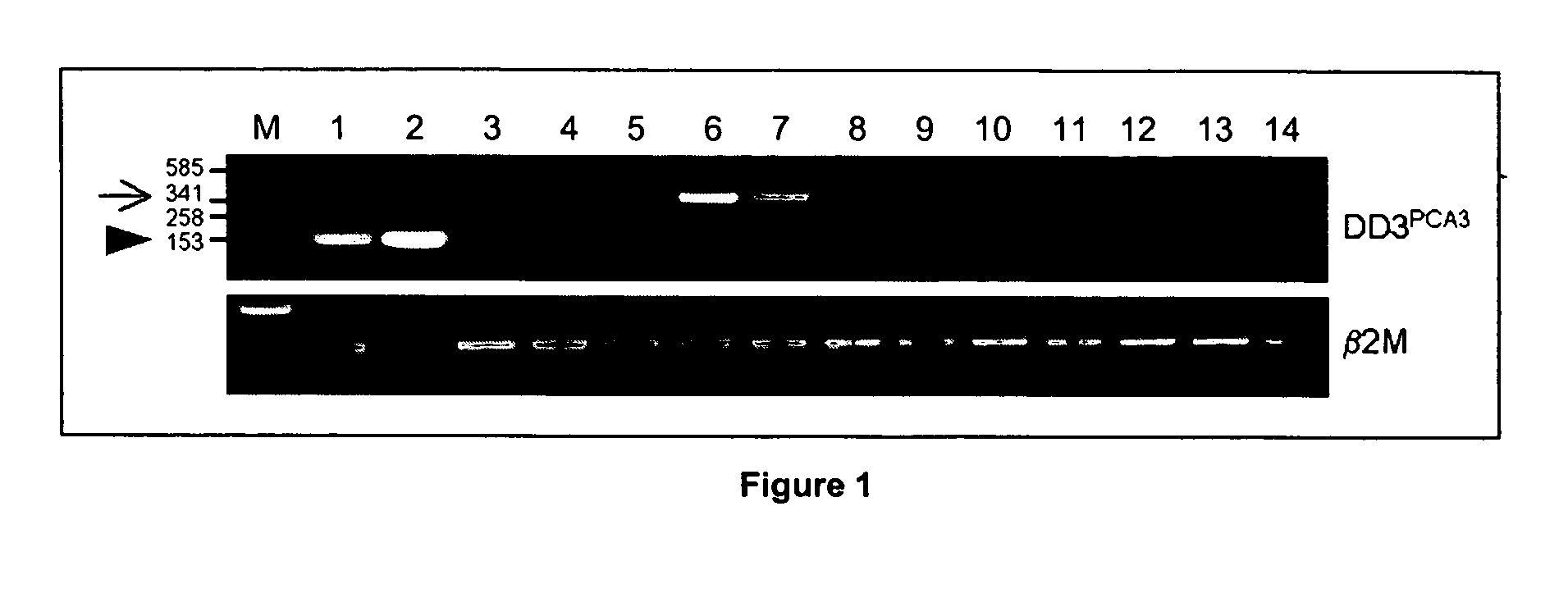
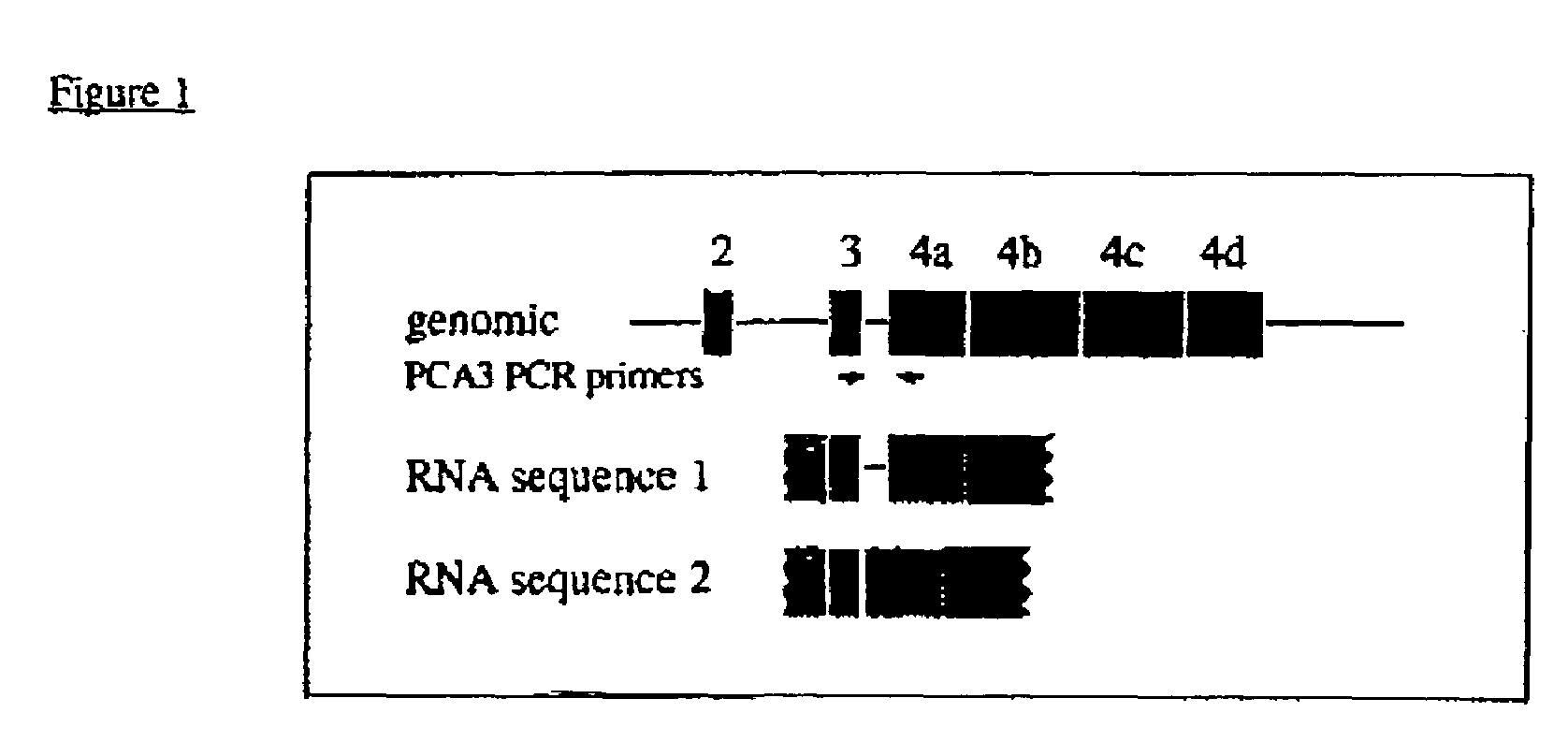
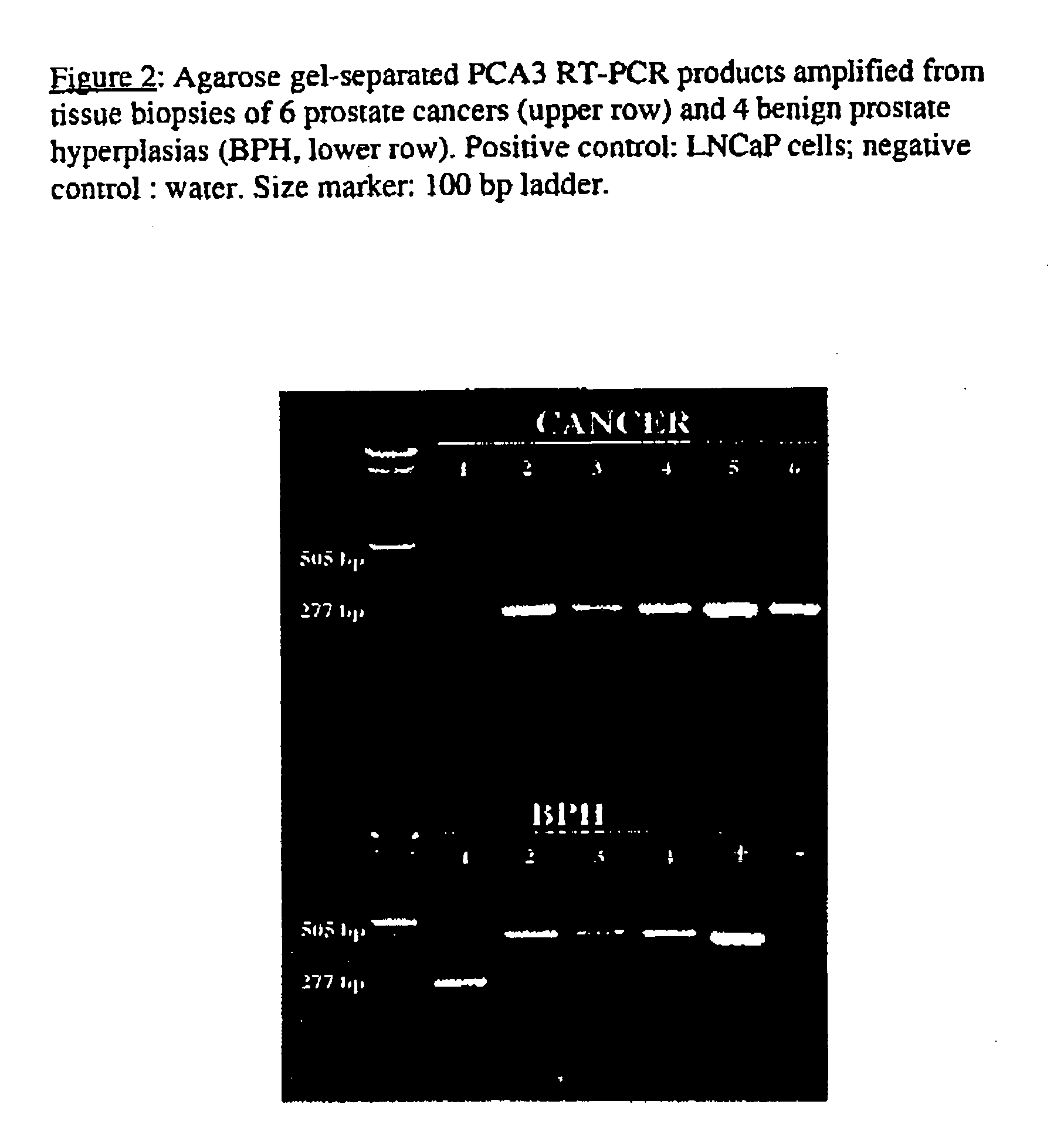
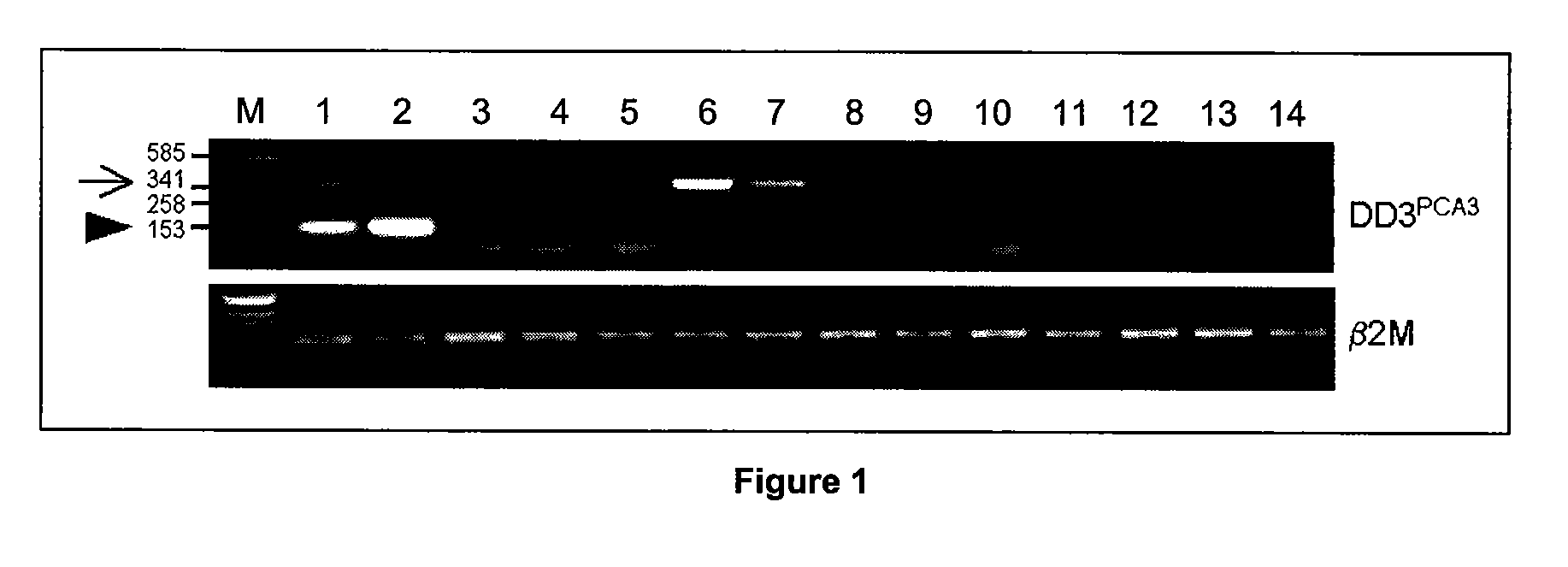
The present invention relates, in general, to prostate cancer. More specifically, the present invention relates to a method to diagnose prostate cancer in a patient by detecting a PCA3 sequence, and more particularly a PCA3 RNA, the PCA3 sequence detected in a sample from the patient being specifically associated with prostate cancer. In a particular embodiment the method and kit enables an amplification of a PCA3 RNA through an exon-exon junction of a spliced PCA3 mRNA. The invention also related to methods and kits to detect such an amplified PCA3 RNA, using a probe which spans the amplified exon-exon junction. The invention also relates to kits containing nucleic acid primers and kits containing nucleic acid primers and nucleic acid probes to diagnose, assess, or prognose a human afflicted with prostate cancer. In particular the methods and kits are designed to detect a PCA3 RNA which lacks one intron or more, and in particular case is intron-less.
Owner:STICHTING KATHOLIEKE UNIV
Method to detect prostate cancer in a sample
InactiveUS20050282170A1Easy to separateConsistent resultMicrobiological testing/measurementNucleotideNucleic acid sequencing
The present invention relates to prostate cancer. More specifically, the present invention relates to a method to detect prostate cancer in a patient sample by detecting the RNA encoded by the gene PCA3. More particularly the present invention relates to a method for determining a predisposition, or presence of prostate cancer in a patient comprising: (a) contacting a biological sample of a patient with at least one oligonucleotide that hybridizes to a PCA3 polynucleotide; (b) detecting in the biological sample an amount of PCA3 and second prostate specific polynucleotides; and (c) comparing the amount of PCA3 polynucleotide that hybridizes to the oligonucleotide to a predetermined cut off value, and therefrom determining the presence or absence of prostate cancer in the biological sample. The present invention further relates to diagnostic kits for the detection of prostate cancer or the risk of developing same in a patient comprising: (a) at least one container means having disposed therein at least one oligonucleotide probe or primer that hybridizes to one a PCA3 nucleic acid or complement thereof; (b) at least one oligonucleotide probe or primer that hybridizes with a second prostate specific nucleic acid or complement thereof; and (c) reagents enabling a detection of PCA3 and of the second prostate specific nucleic acid when PCA3 or second prostate-specific nucleic acid sequence is present.
Owner:GEN PROBE INC
Nucleic acid molecules comprising the promoter for PCA3, and uses thereof
InactiveUS6897024B2Overcomes drawbackInhibit progressBacteriaSugar derivativesGene expressionSugar nucleotide
This invention describes the cloning and characterization of the promoter region for the PCA3dd3 gene. This region regulates the PCA3dd3 gene expression by a unique prostate specific transcriptional mechanism. The present invention relates to the use of this promoter region as a tool for prostate cancer treatment and diagnosis and screening of agents which regulate expression of PCA3. In a particular embodiment, the present invention relates to an isolated promoter sequence which comprises an isolated promoter sequence which comprises a sequence as set forth between nucleotide positions 372 to 460 of SEQ ID NO:1, this sequence enabling a prostate-specific modulation of transcription.
Owner:STICHTING KATHOLIEKI UNIV MORE PARTICULARLY THE UNIV MEDICAL CENT NIJMEGEN
Nucleic acid molecules comprising the promoter for PCA3dd3, a new prostate antigen, and uses thereof
InactiveUS20050158792A1Overcomes drawbackInhibit progressSugar derivativesBacteriaGene expressionSugar nucleotide
This invention describes the cloning and characterization of the promoter region for the PCA3dd3 gene. This region regulates the PCA3dd3 gene expression by a unique prostate specific transcriptional mechanism. The present invention relates to the use of this promoter region as a tool for prostate cancer treatment and diagnosis and screening of agents which regulate expression of PCA3. In a particular embodiment, the present invention relates to an isolated promoter sequence which comprises an isolated promoter sequence which comprises a sequence as set forth between nucleotide positions 372 to 460 of SEQ ID NO:1, this sequence enabling a prostate-specific modulation of transcription.
Owner:STICHTING KATHOLIEKE UNIV
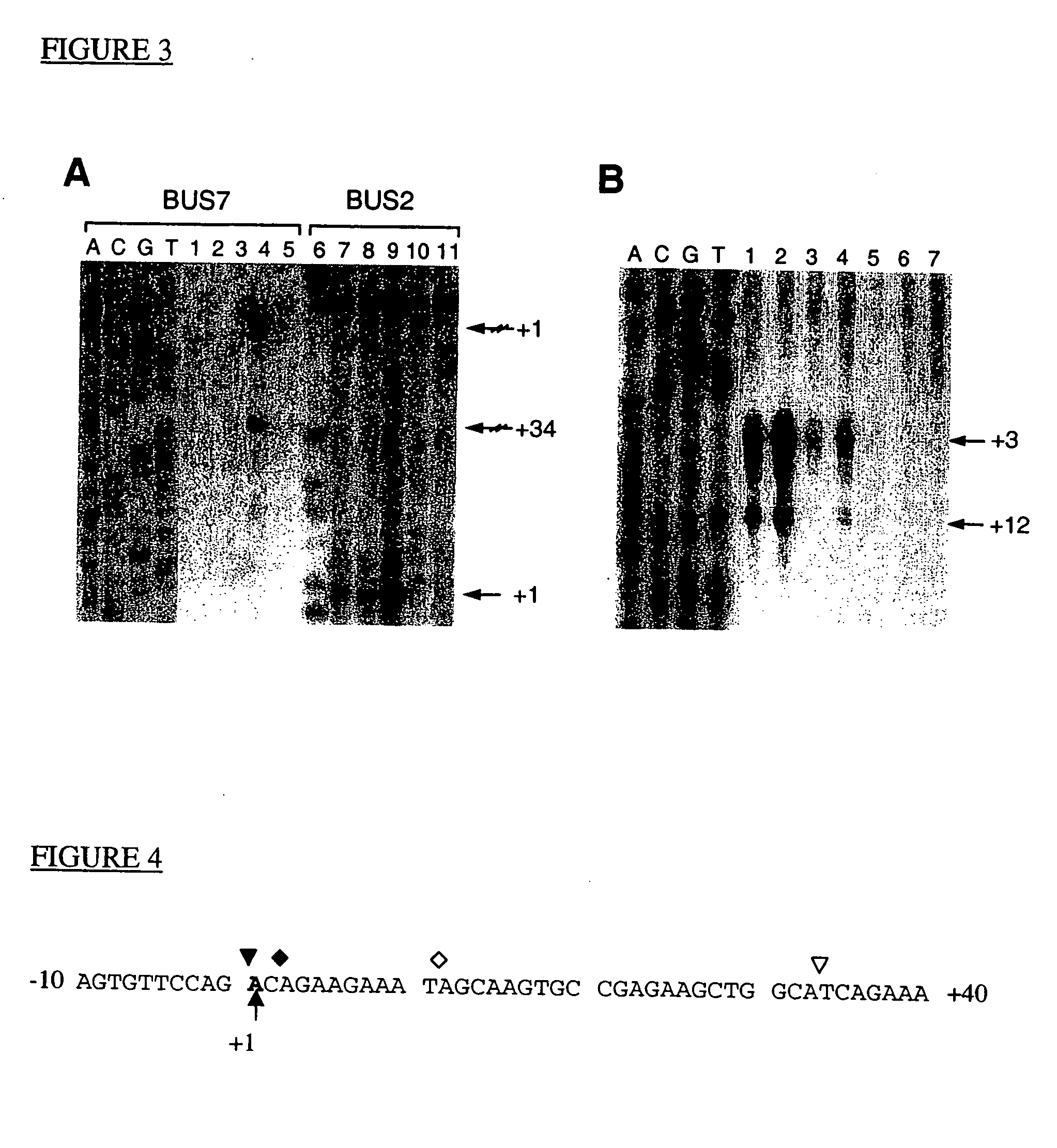
PCA3 messenger RNA species in benign and malignant prostate tissues
InactiveUS7368545B1High detection sensitivityHigh sensitivityOrganic active ingredientsFungiNon malignantMessenger RNA
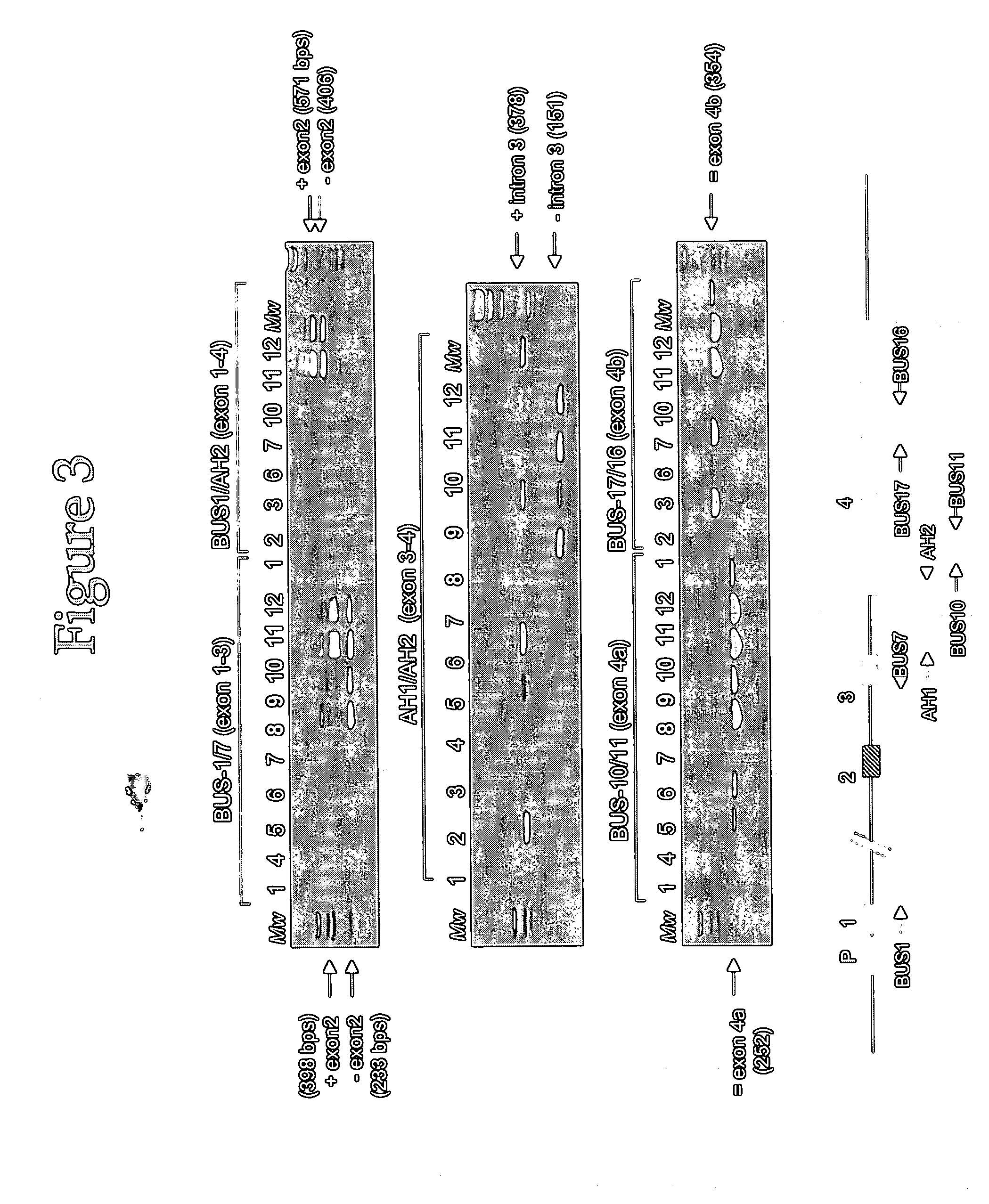
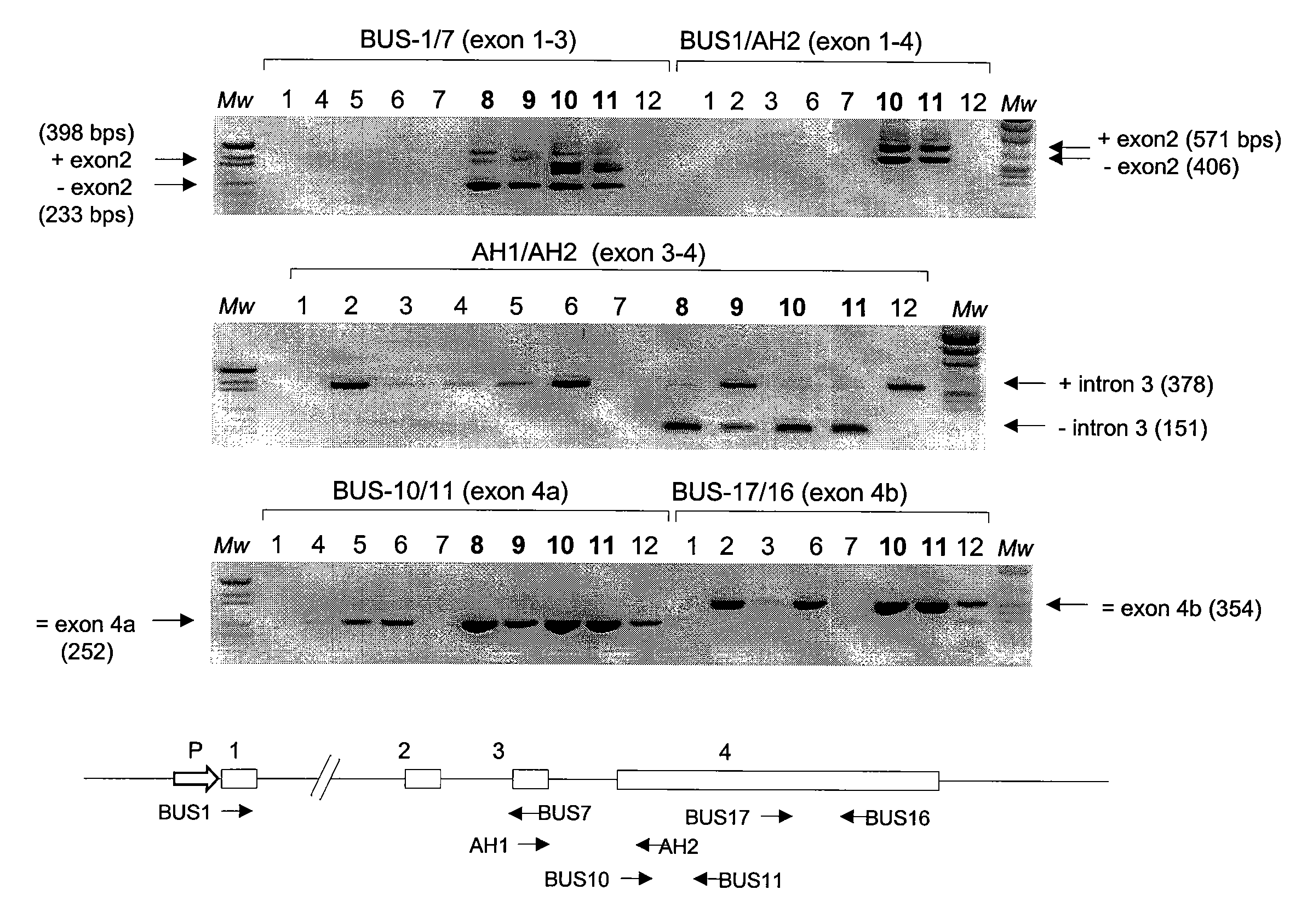
This invention concerns the discovery of two distinct PCA3 mRNA sequences. One of these sequences corresponds to a short PCA3 mRNA molecule whereas the other PCA3 RNA molecule is longer as it comprises an additional sequence between exon 3 and exon 4a. The short RNA is associated with prostate cancer whereas the long RNA sequence is associated with a non-malignant state of the prostrate. Based on the differential expression levels of these two PCA3 RNA sequences, protocols for the diagnosis of prostate disease are provided. The invention also relates to therapeutic approaches to prostate cancer.
Owner:GEN PROBE INC
mRNA ratios in urinary sediments and/or urine as a prognostic and/or theranostic marker for prostate cancer
ActiveUS7960109B2Microbiological testing/measurementBiological material analysisBacteriuriaUrinary sediment
Owner:STICHTING KATHOLIEKE UNIV
PCA3, PCA3 genes, and methods of use
The present invention relates, in general, to a prostate-specific antigen, PCA3. In particular, the present invention relates to nucleic acid molecules coding for the PCA3 protein; purified PCA3 proteins and polypeptides; recombinant nucleic acid molecules; cells containing the recombinant nucleic acid molecules; antibodies having binding affinity specifically to PCA3 proteins and polypeptides; hybridomas containing the antibodies; nucleic acid probes for the detection of nucleic acids encoding PCA3 proteins; a method of detecting nucleic acids encoding PCA3 proteins or polypeptides in a sample; kits containing nucleic acid probes or antibodies; bioassays using the nucleic acid sequence, protein or antibodies of this invention to diagnose, assess or prognose a mammal afflicted with prostate cancer; therapeutic uses; and methods of preventing prostate cancer in an animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Gene expression panel for prognosis of prostate cancer recurrence
ActiveUS20160333420A1Health-index calculationMicrobiological testing/measurementABCC11Clinical recurrence
Disclosed is a gene expression panel that can be used to predict prostate cancer (PCa) progression. Some embodiments provide methods for predicting clinical recurrence of PCa. Some embodiments provide a method for predicting progression of prostate cancer in an individual, the method comprising: (a) receiving expression levels of a collection of signature genes from a biological sample taken from said individual, wherein said collection of signature genes comprises at least two genes selected from the group consisting of: NKX2-1, UPK1A, ADRA2C, ABCC11, MMP11, CPVL, ZYG11A, CLEC4F, OAS2, PGC, UPK3B, PCBP3, ABLIM1, EDARADD, GPR81, MYBPC1, F10, KCNA3, GLDC, KCNQ2, RAPGEF1, TUBB2B, MB, DUOXA1, C2orf43, DUOX1, PCA3 and NPR3; (b) applying the expression levels to a predictive model relating expression levels of said collection of signature genes with prostate cancer progression; and (c) evaluating an output of said predictive model to predict progression of prostate cancer in said individual. Systems are also provided for predicting progression and / or recurrence of PCa.
Owner:ILLUMINA INC +1
Methods, kits and compositions for providing a clinical assessment of prostate cancer
InactiveCN104603292AHealth-index calculationMicrobiological testing/measurementExpression geneOncology
The present invention relates to prostate cancer signatures which are useful for providing a clinical assessment of prostate cancer from a biological sample of a subject. By performing initial gene expression studies on urine samples from prostate cancer and non-prostate cancer subjects, and using the PCA3 / PSA prostate cancer test as a performance benchmark, the present inventors have surprisingly discovered multiple signatures that are informative in urine-based prostate cancer tests, as well as in tissue-based tests. The signatures relate to combinations of at least two prostate cancer markers whose expression pattern in urine has been validated as being associated (either positively or negatively) with a clinical assessment of prostate cancer. The prostate cancer markers can be used in conjunction with bioinformatics approaches to generate a prostate cancer score, which correlates with a clinical assessment of prostate cancer. Methods, kits and compositions relating to the aforementioned signatures are also described.
Owner:DIAGNOCURE
Primer pairs, probes and kit for early diagnosis of prostatic cancer (PC)
ActiveCN102912030AHigh sensitivityLow costMicrobiological testing/measurementDNA preparationBiotin-streptavidin complexNucleotide
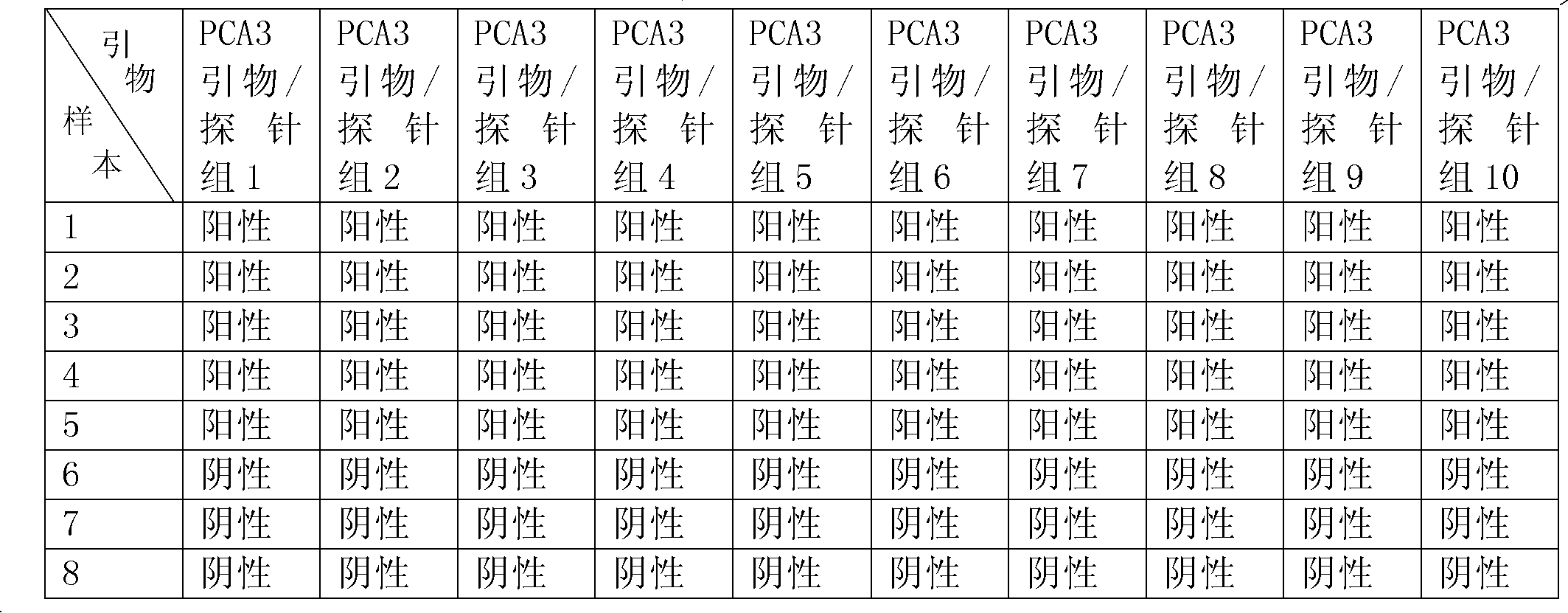
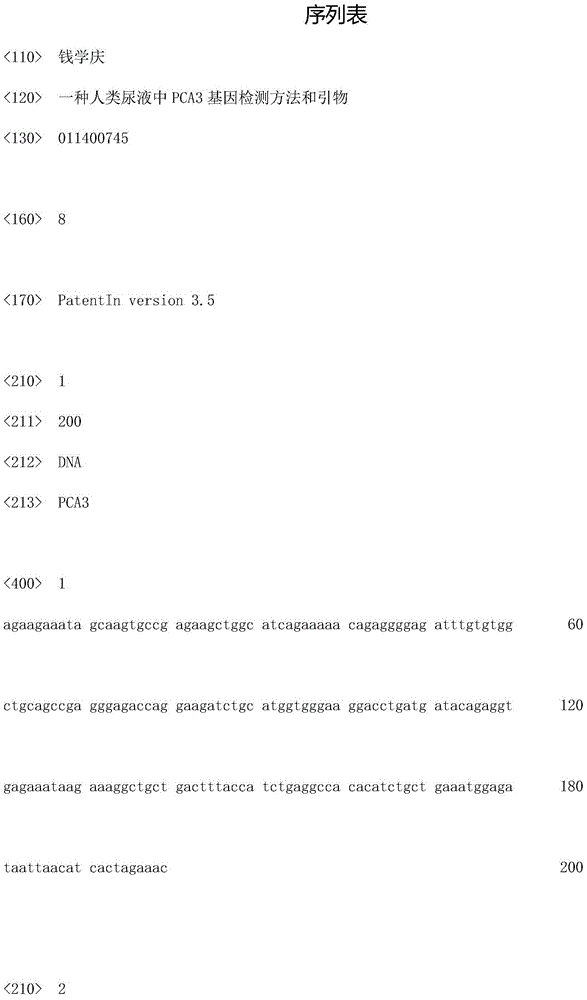
The invention belongs to the immunology field and relates to three groups of primer pairs and probes and a kit for early diagnosis of the PC. The three groups of the primer pairs are provided with nucleotide sequences shown by SEQ ID NO: 1and SEQ ID NO: 4, SEQ ID NO: 2 and SEQ ID NO: 5 and SEQ ID NO: 3 and SEQ ID NO: 6 respectively. Three DNA probes are marked with biotin at 3' ends and are provided with nucleotide sequences shown by SEQ ID NO: 7, SEQ ID NO: 8 and SEQ ID NO: 9, and luminophore is connected on amino acid radicals at * positions in the sequences. The kit for early diagnosis of the PC is provided with the three groups of the primer pairs, reverse transcriptase and RNA polymerase and streptavidin coated on the RNA polymerase, and solid phase carriers of the DNA probes are connected onto the streptavidin. According to primer pairs, probes and the kit for early diagnosis of the PC, expression levels of human body TMP RSS2: ERG, PCA3 and PSA genes to judge risks of the PC through transcription-mediated amplification techniques.
Owner:端鹏 +2
Liquid-phase chip method for detecting PCA3 gene and PSA gene and diagnostic reagent kit thereof
ActiveCN101942523AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseBiotin-streptavidin complex
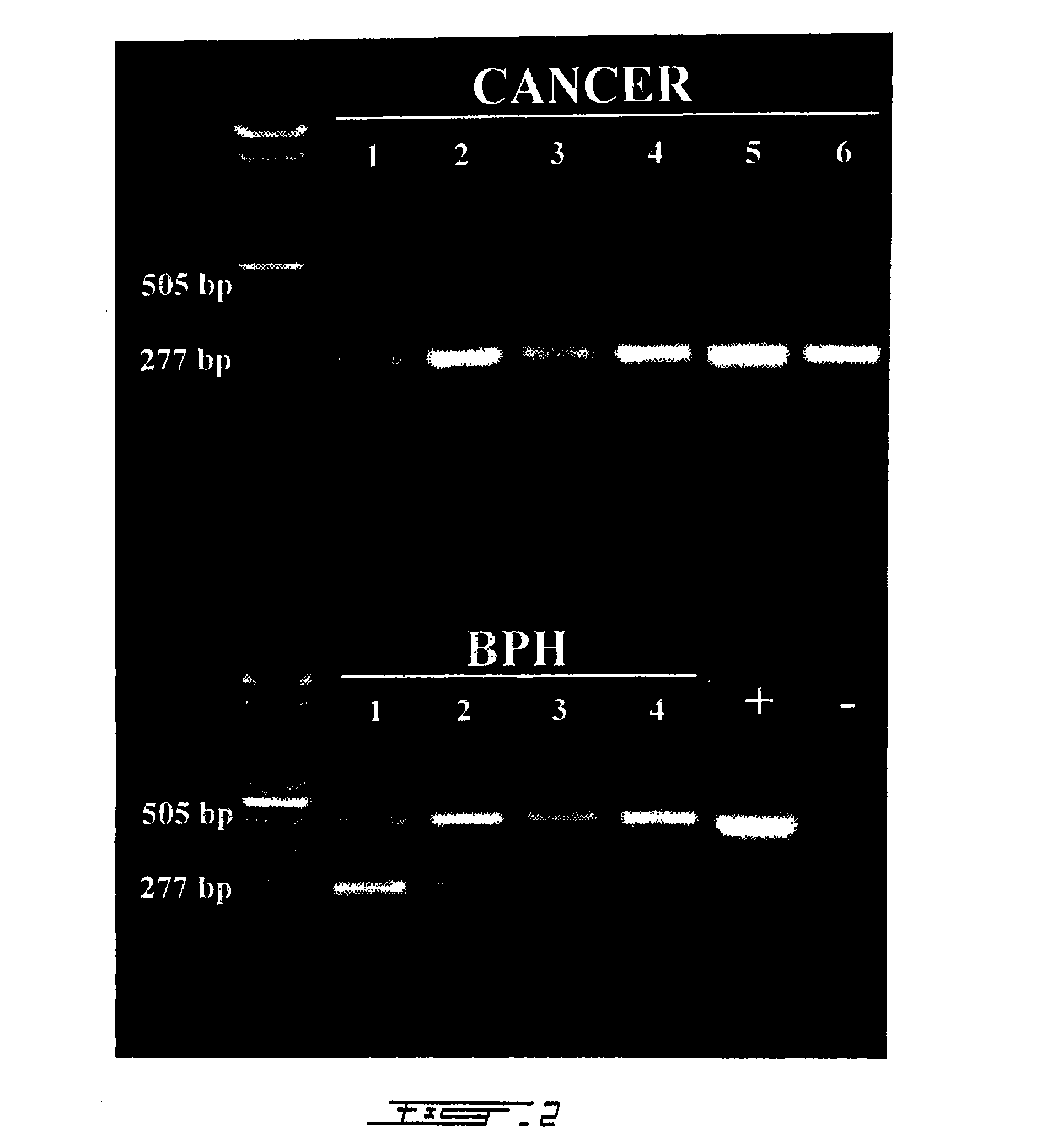
The invention discloses a liquid-phase chip method for detecting a PCA3 gene and a PSA gene and a diagnostic reagent kit thereof. A primer and a probe are designed for mRNA of the PCA3 gene and / or the PSA gene; a mixture, formed by the covalent bonding of the probe and microsphere, is hybridized with a reverse transcription P CR amplification product, after streptavidin phycoerythrin is added fluorescent signals of different microspheres can be detected to determine whether a sample to be detected contains the PCA3 and PSA genes and the expression condition thereof, and simultaneously, the PCA3 / PSA ratio can be obtained. The liquid-phase chip method and the reagent kit have the advantages of high sensitivity, high throughput, quick and accurate detection and the like, and can perform qualitative and quantitative detection on the PCA3 and PSA genes related to different degrees of prostate diseases including prostate cancer and benign prostatic hyperplasia and perform specific diagnosison the prostate cancer.
Owner:邵棠
PCA3 Messenger RNA Species in Benign and Malignant Prostate Tissues
InactiveUS20080261228A1High detection sensitivityHigh sensitivityOrganic active ingredientsFungiNon malignantMessenger RNA
Owner:GEN PROBE INC
PCA3 gene detection method and primer in human urine
InactiveCN104531863AConducive to early treatmentAvoid damageMicrobiological testing/measurementDNA/RNA fragmentationTotal rnaUrine production
The invention relates to the technical field of gene detection and provides a method for extracting total RNA from urine of a subject and detecting PCA3 mRNA and PSA mRNA and a primer for performing specific amplification on PCA3 mRNA and PSA mRNA. The primer comprises two groups of PCR amplified nucleic acid primers for detecting the PCA3 mRNA and PSA mRNA, the length of each primer is 18-26bp, and the PCA3 mRNA fragment and PSA mRNA fragment are respectively and specifically amplified. According to the amplification result, the ratio of PCA3 mRNA to PSA mRNA is measured, and the PCA3 grade is calculated, so that the aims of realizing early diagnosis of prostatic cancer and reducing prostate puncture as much as possible are achieved.
Owner:上海润达榕嘉生物科技有限公司
Application of BDNA for detecting PCA3 in urine
InactiveCN103436594AAvoid false positivesAvoiding the False Negative ProblemMicrobiological testing/measurementSmall fragmentTrue positive rate
The invention provides an application of BDNA for detecting PCA3 in urine. Operation steps comprise (1) collecting PCA3 specimens; (2) studying the collection of the PCA3 specimens; (3) detecting with a BDNA technology. By utilizing the bDNA technology being good at capture of small fragment genes, efficiently specific combination of uniquely designed target probes and PCA3 and an improved signal amplifying system, both sensitivity and specificity are guaranteed. The technology prevents the problems of false positive and false negative of conventional PCR, is simple in operations, and can be operated by a person with basic skills of molecular biology experiments. Meanwhile, the technology has high detected result sensitivity, high positive detectable rate and good stability and repeatability, is convenient for operations, is time-saving and can be popularized easily.
Owner:KODIA XINXIANG BIO TECH
Specific method of prostate cancer detection based on pca3 gene, and kits therefor
InactiveUS20090233285A1Strong specificityHigh sensitivityMicrobiological testing/measurementExonBiology
The present invention relates, in general, to prostate cancer. More specifically, the present invention relates to a method to diagnose prostate cancer in a patient by detecting a PCA3 sequence, and more particularly a PCA3 RNA, the PCA3 sequence detected in a sample from the patient being specifically associated with prostate cancer. In a particular embodiment the method and kit enables an amplification of a PCA3 RNA through an exon-exon junction of a spliced PCA3 mRNA. The invention also relates to methods and kits to detect such an amplified PCA3 RNA, using a probe which spans the amplified exon-exon junction. In particular the methods and kits are designed to detect a PCA3 RNA which lacks one intron or more, and in particular case is intron-less. The invention further relates to a method of detecting PCA3 RNA expressed in non-prostate tissue or cells of the urinary tract, that comprises PCA3 intron 3.
Owner:STICHTING KATHOLIEKE UNIV
Urine nucleic acid maker and detection reagent kit for assisting in early screening of prostatic cancer
ActiveCN111778331ASimplify the screening processImprove reliabilityMicrobiological testing/measurementDNA/RNA fragmentationReference genesProstate cancer screening
The invention discloses a urine nucleic acid maker and detection reagent kit for assisting in early screening of prostatic cancer. The nucleic acid marker comprises three prostatic cancer related genes of a T2E fusion gene, a PCA3 gene and an MMP-2 gene, and two internal reference genes of a PSA gene and an SPDEF gene. A multiple fluorescence reverse transcription polymerase chain reaction (RT-PCR) detection reagent comprising the five nucleic acid markers can be used for assisting in screening of prostatic cancer, an area under curve (AUC) of a testee operating characteristic (ROC) curve canachieve 0.81, and sensitivity predicted value and negative predicted value are respectively as high as 91.6% and 93.3%. The detection reagent kit is simple to operate, and quantitative detection of the nucleic acid markers and screening of the prostatic cancer can be completed in one pipe of the reagent. Unnecessary puncturing inspection of prostate can be notably reduced, and the reagent kit hasfavorable clinical application prospects.
Owner:诺迦(杭州)生物工程有限公司
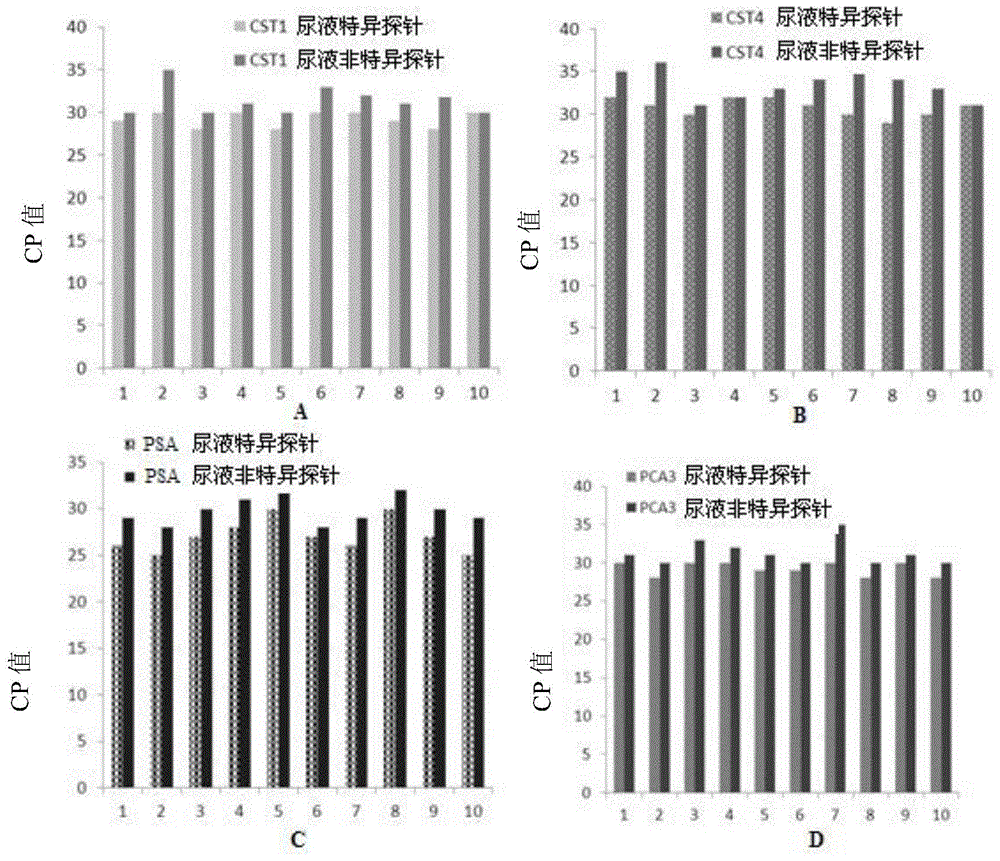
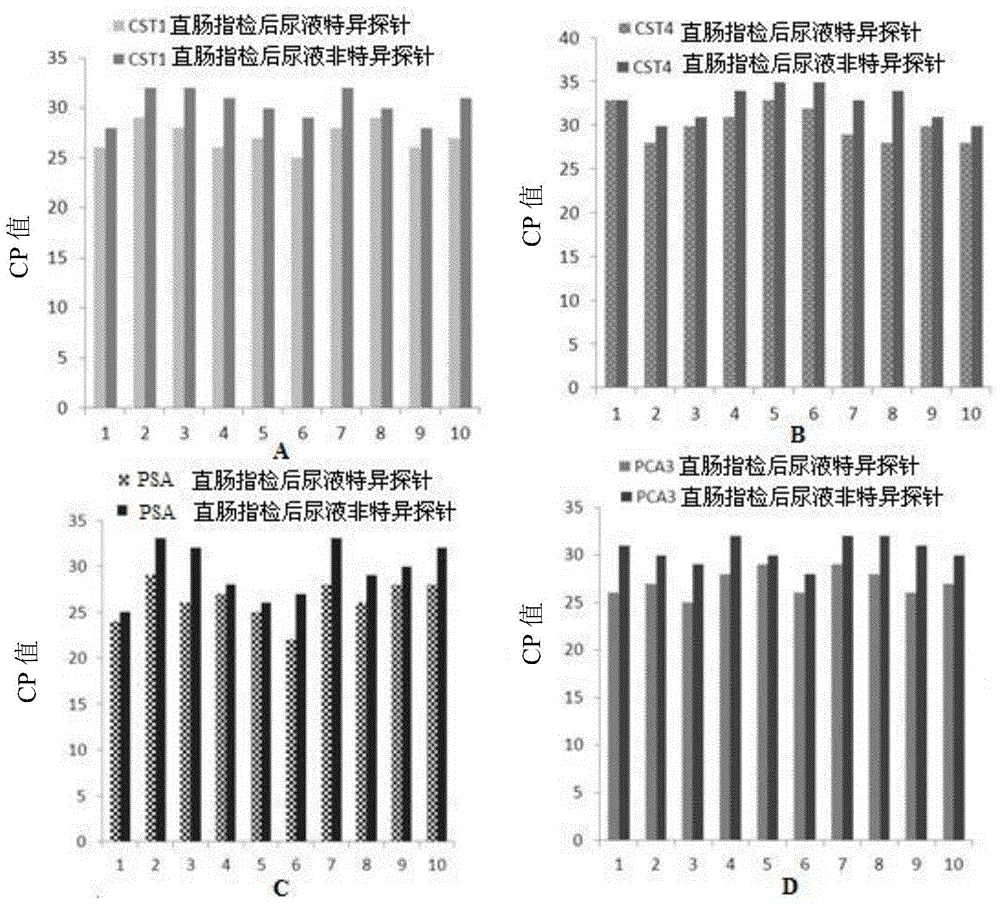
Application of PCA3, CST1 and CST4 to preparation of prostate gland cancer marker and kit of PCA3, CST1 and CST4
ActiveCN103966352AEasy to useLess sampling painMicrobiological testing/measurementBacteriuriaTherapeutic effect
The invention discloses application of PCA3, CST1 and CST4 to preparation of a prostate gland cancer marker and a kit of the PCA3, the CST1 and the CST4. The specificity and the sensitivity of a PCA3, CST1 and CST4 combination for diagnosing and indicating the prostate gland cancer are higher than those of the PCA3 as the marker. The kit is used for combined detection of the marker, is convenient to use, has the characteristics of being good in specificity and high in sensitivity, and can be used for diagnosing the prostate gland cancer, evaluating the treatment effect in a treatment process and monitoring the metastasis and relapse after the treatment; urine can be directly collected for detection without serum collection, so that the pain of patients during sampling is relieved; a guidance is provided for early intervention of doctors.
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
PCA3 and PSA RNA detection kit and amplification system
PendingCN110951871AHigh sensitivityImprove featuresMicrobiological testing/measurementFluoProbesGene selection
The invention relates to a PCA3 and PSA RNA detection kit and amplification system. The detection kit includes primers and fluorescent probes aiming at PCA3 and PSA genes, and a Stoffel fragment and Tfl DNA polymerase; the primers aiming at the PCA3 gene are shown as SEQ ID NO. 1 and SEQ ID NO. 2, and the sequence of the fluorescent probe is shown as SEQ ID NO. 3; and the primers aiming at the PSAgene are shown as SEQ ID NO. 10 and SEQ ID NO. 11, and the sequence of the fluorescent probe is shown as SEQ ID NO. 12. Based on existing kits, the detection and amplification system of real-time fluorescence quantitative RT-PCR can be optimized; the PCA3 gene selects the primers and probes which have better detection effects and are designed by the sequence on exon 3; optimized Tris-MOPS-sodiumcitrate buffer which is more suitable for RNA amplification can be introduced into a reaction system, and the Stoffel fragment or the Tfl DNA polymerase is introduced as well; and therefore, the kit can tolerate more templates while detection sensitivity and specificity are enhanced.
Owner:RAYBIOTECH INC GUANGZHOU
Non-invasive method for diagnosis of prostate cancer
InactiveUS20130035250A1Improve diagnostic reliabilityImprove reliabilityMicrobiological testing/measurementLibrary screeningBacteriuriaEZH2
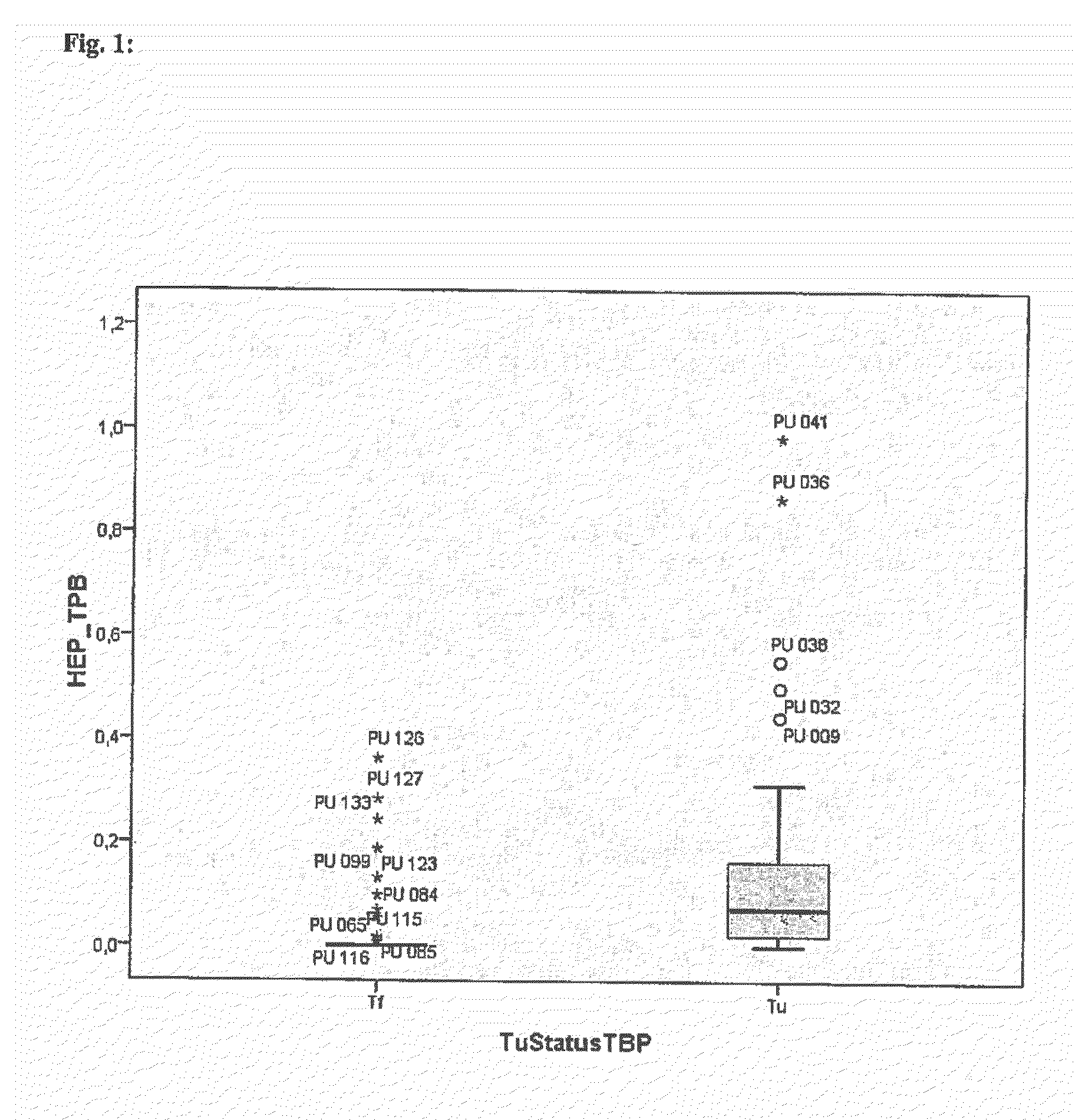
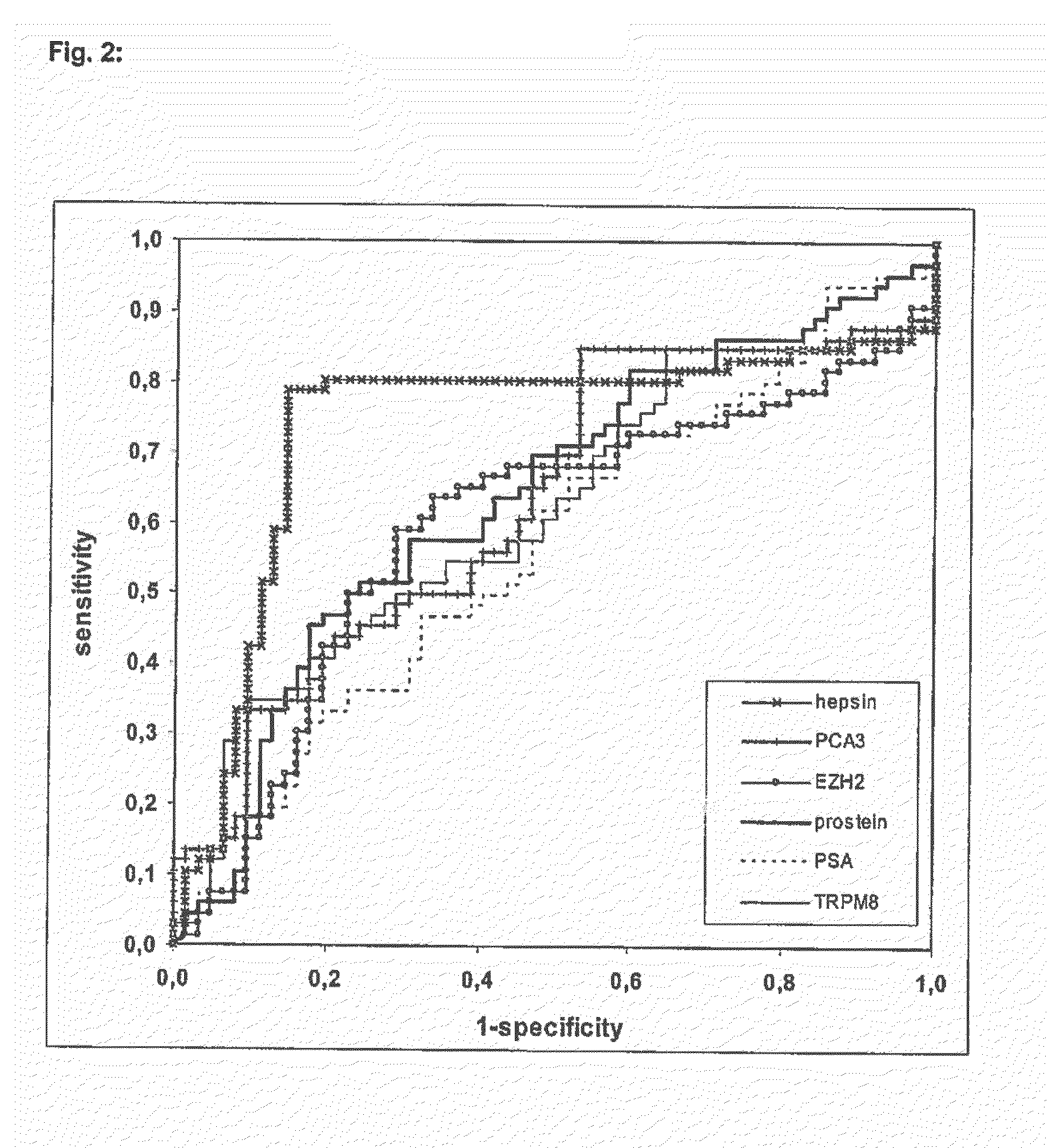
The present invention relates on a non-invasive method for diagnosing prostate cancer and / or assessing the risk of a subject acquiring prostate cancer comprising the analysis of the expression of the marker gene hepsin in an urine sample. It further relates on a non-invasive method for diagnosing prostate cancer and / or assessing the risk of a subject acquiring prostate cancer by determining the expression levels of the marker genes hepsin, EZH2, prostein and PCA3.
Owner:DRESDEN UNIVERSITY OF TECHNOLOGY
Prostate cancer detection probe sequence and application thereof
ActiveCN108504738AStrong specificityBest limit of quantitationMicrobiological testing/measurementDNA/RNA fragmentationProstate cancer cellMedicine
The invention relates to the technical field of genetic engineering and discloses a prostate cancer detection probe sequence and the application thereof. The probe sequence comprises a capturing extension probe, a K-type marking extension probe and a sealing probe. The capturing extension probe, the K-type marking extension probe and the sealing probe are designed and artificially optimized for prostate cancer PCA3, the specificity of prostate cancer cell detection based on similar bDNA and QuantiMAT techniques can be improved, relatively good quantitative limits, detection limits and sensitivity can be achieved, and the probe can be applied to preparation of kits.
Owner:ZHENGZHOU KODIA BIOTECHNOLOGY CO LTD
Analytical method for identifying prostate cancer based on urine exosomes
The invention discloses an analytical method for identifying prostate cancer based on urine exosomes in the field of prostate cancer. According to the analytical method for identifying prostate cancerbased on the urine exosomes,the exosomes are directly extracted from human urine, whether the prostate cancer is diagnosed or not is identified by measuring the content of PCA3, ERG group, and SPDEFin the exosomes,the accuracy of the identification result is higher than that of a urine detection kit adopting PCA3 / PSA, and the detection can be completed by using only ordinary PCR instruments without the need to purchase additional equipment.
Owner:赵凯
Risk assessment device for prostate cancer
PendingCN113528660AAvoid initial biopsyAvoid pollutionMicrobiological testing/measurementOncologyProstatic Cancers
The invention provides a risk assessment device for prostate cancer. The device comprises a collection device; a nucleic acid extraction device; an amplification device and a data acquisition and analysis device, wherein the data acquisition and analysis device is used for quantitatively detecting expression quantities of a PCA3 gene, an ERG gene and an SPDEF gene in a sample RNA solution and calculating expression quantities of the PCA3 gene and the ERG gene relative to the SPDEF gene, wherein delta CT of the PCA3 gene and the ERG gene is calculated by recording Ct values of the PCA3 gene, the ERG gene and the SPDEF gene, the sum of the relative expression quantities of the PCA3 gene and the ERG gene is calculated by using a calculation formula of the relative expression quantities, namely delta delta CT = 2^delta Ct (PCA3) + 2^delta Ct (ERG), and then a score is calculated by using a formula (16.92-delta delta Ct) * 1.83 and can reflect the risk level of the prostatic cancer.
Owner:SHENZHEN HUIXIN LIFE TECH CO LTD
Application of pca3, cst1 and cst4 in preparation of prostate cancer markers and kit thereof
ActiveCN103966352BEasy to useLess sampling painMicrobiological testing/measurementBacteriuriaLymphatic Spread
Owner:SHANGHAI LIANGRUN BIOMEDICINE TECH CO LTD
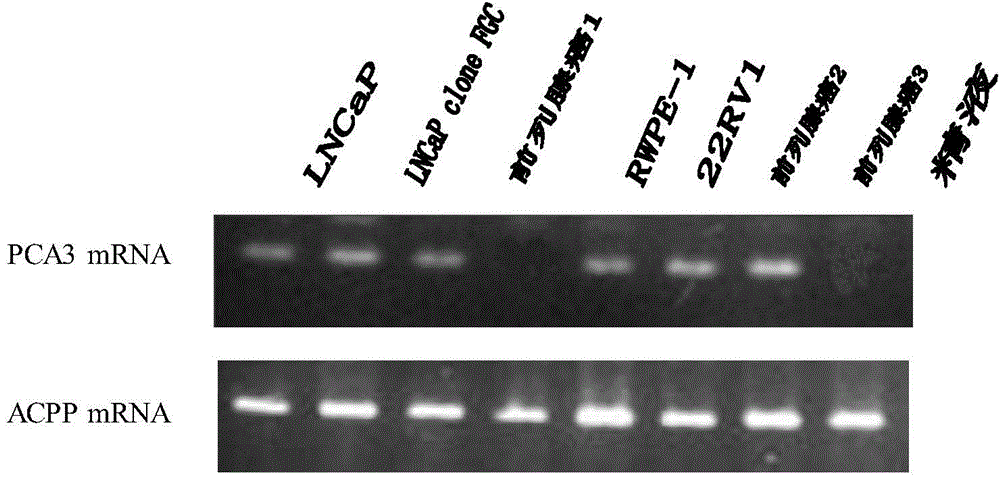
PCA3 mRNA/ACPP mRNA RT-PCR detection primer and detection kit thereof
InactiveCN104878077AImprove diagnostic accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBiologyPcr dgge
The invention provides a PCA3 mRNA / ACPP mRNA RT-PCR detection primer and a detection kit thereof. The detection primer comprises an upstream primer represented by 5'-CTCCTCAACATGAGAGCTGC-3' and a downstream primer represented 5'-TATATACTCTCCAAGTTCATA-3'. The detection kit comprises an RT-PCR reaction solution, MMLV RT / Hot start Taq Mix, a positive reference substance and a negative reference substance. The PCA3 mRNA / ACPP mRNA RT-PCR detection kit prepared in the invention is better than PCA3 mRNA / PSA mRNA RT-PCR combination detection kits, and the detection sensitivity of the detection kit prepared in the invention increases to above 90%.
Owner:谭巍
Gene expression panel for prognosis of prostate cancer recurrence
ActiveUS10364469B2Health-index calculationMicrobiological testing/measurementABCC11Clinical recurrence
Disclosed is a gene expression panel that can be used to predict prostate cancer (PCa) progression. Some embodiments provide methods for predicting clinical recurrence of PCa. Some embodiments provide a method for predicting progression of prostate cancer in an individual, the method comprising: (a) receiving expression levels of a collection of signature genes from a biological sample taken from said individual, wherein said collection of signature genes comprises at least two genes selected from the group consisting of: NKX2-1, UPK1A, ADRA2C, ABCC11, MMP11, CPVL, ZYG11A, CLEC4F, OAS2, PGC, UPK3B, PCBP3, ABLIM1, EDARADD, GPR81, MYBPC1, F10, KCNA3, GLDC, KCNQ2, RAPGEF1, TUBB2B, MB, DUOXA1, C2orf43, DUOX1, PCA3 and NPR3; (b) applying the expression levels to a predictive model relating expression levels of said collection of signature genes with prostate cancer progression; and (c) evaluating an output of said predictive model to predict progression of prostate cancer in said individual. Systems are also provided for predicting progression and / or recurrence of PCa.
Owner:ILLUMINA INC +1
Prostate cancer diagnosis and prognosis evaluation kit
PendingCN113025721AHigh sensitivityImprove featuresMicrobiological testing/measurementLibrary creationChromosome StabilityChromosome instability
The invention provides a group of combination of a chromosome instability region and a gene variation region. The chromosome instability region and the gene variation region comprise 2q, 3q, 5q, 6q, 7p, 7q, 8p, 8q, 9p, 12q, 13q, 14q, 16q, 18q, PCA3 and ERG. The content of 16 chromosome unstable regions and gene variation regions in prostate cancer patients is as high as 91.5%, and when being applied to reagents or kits for screening and / or diagnosing and / or prognostically evaluating prostate cancer, the very high sensitivity and specificity and very good clinical application prospect can be achieved.
Owner:SUZHOU HONGYUAN BIOTECH CO LTD
Application of exosome gene, prostate cancer detector, and detection kit and detection device thereof
ActiveCN113249481AMicrobiological testing/measurementMedical automated diagnosisBiologic markerFusion gene
The invention relates to application of an exosome gene, a prostate cancer detector, and a detection kit and a detection device of the prostate cancer detector. The application of the exosome gene as a biomarker in preparation of a prostate cancer detection reagent, a prostate cancer detection kit or a prostate cancer detection device is characterized in that the exosome gene contains at least one of a PCA3 gene, an SPDEF gene and a TMPRSS2: ERG fusion gene. The biomarker is used for preparing a prostate cancer detection reagent, a prostate cancer detection kit or a prostate cancer detection device, and the positive detection rate of prostate cancer is high.
Owner:深圳市拾方杰科技有限公司
Urine detection kit for prostate cancer and application thereof
ActiveCN103849683BEasy to operateShort timeMicrobiological testing/measurementAntigenProstate-specific antigen
The invention discloses a urine detection kit for prostate cancer. The kit comprises a one-step method RT-PCR (Reverse Transcription-Polymerase Chain Reaction) amplification reagent which comprises specific primers for detecting PCA (Prostate Cancer Antigen) 3 and PSA (Prostate Specific Antigen) and a probe. The primers for detecting the PCA3 are as shown in SEQ ID NO.1 and SEQ ID NO.2, and the probe is as shown in SEQ ID NO.5. The primers for detecting the PSA are as shown in SEQ ID NO.3 and SEQ ID NO.4, and the probe is as shown in SEQ ID NO.6. The kit has the advantages of simple and convenient operational process, short consumed time, simplicity in result judgment, good repeatability and low recheck rate, and after a template is added, the whole course of the PCR reaction is carried out in a closed tube, so that a false positive phenomenon caused by pollution is avoided.
Owner:JIANGSU MICRODIAG BIOMEDICINE TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com