Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Cardiac repair" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
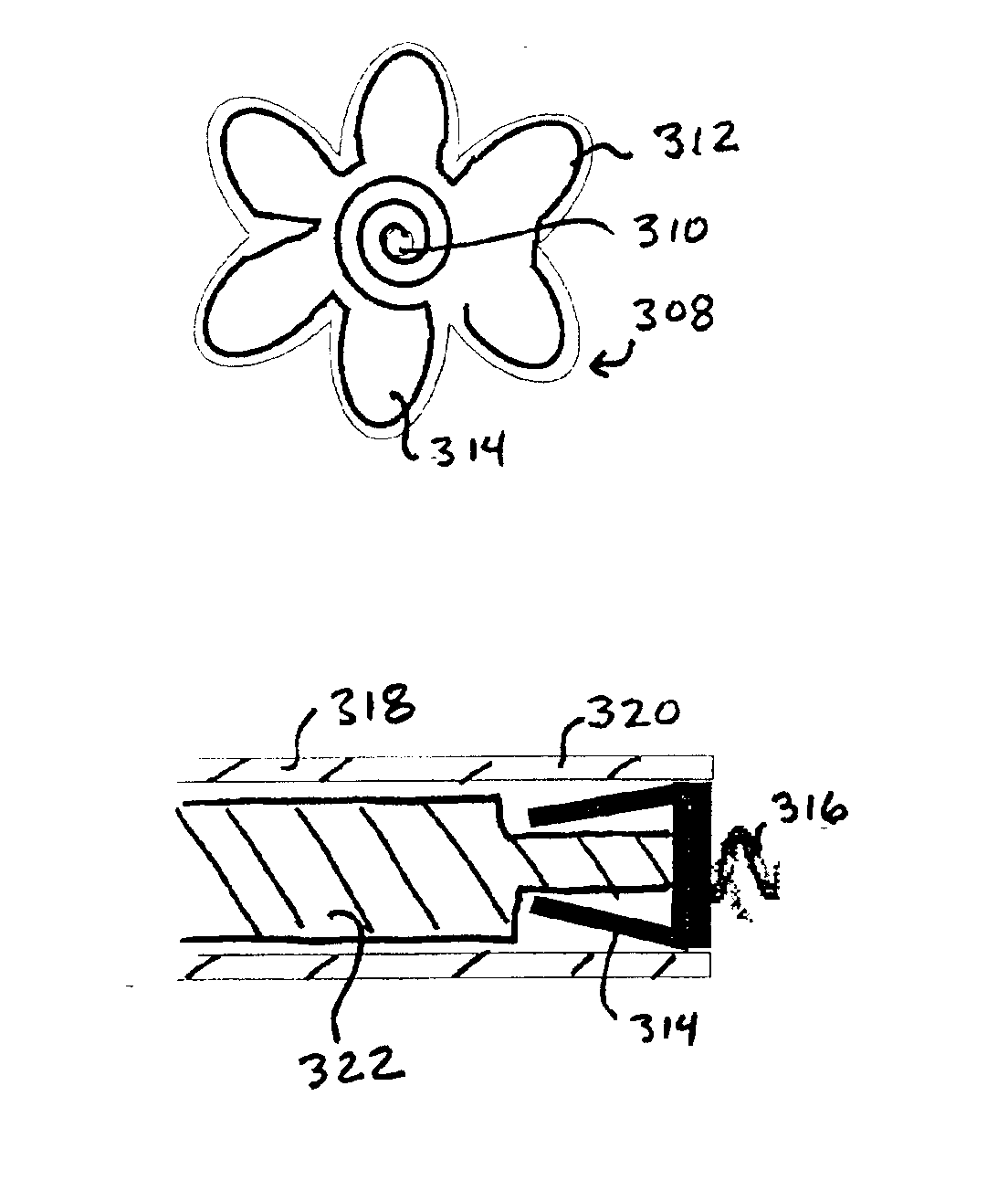
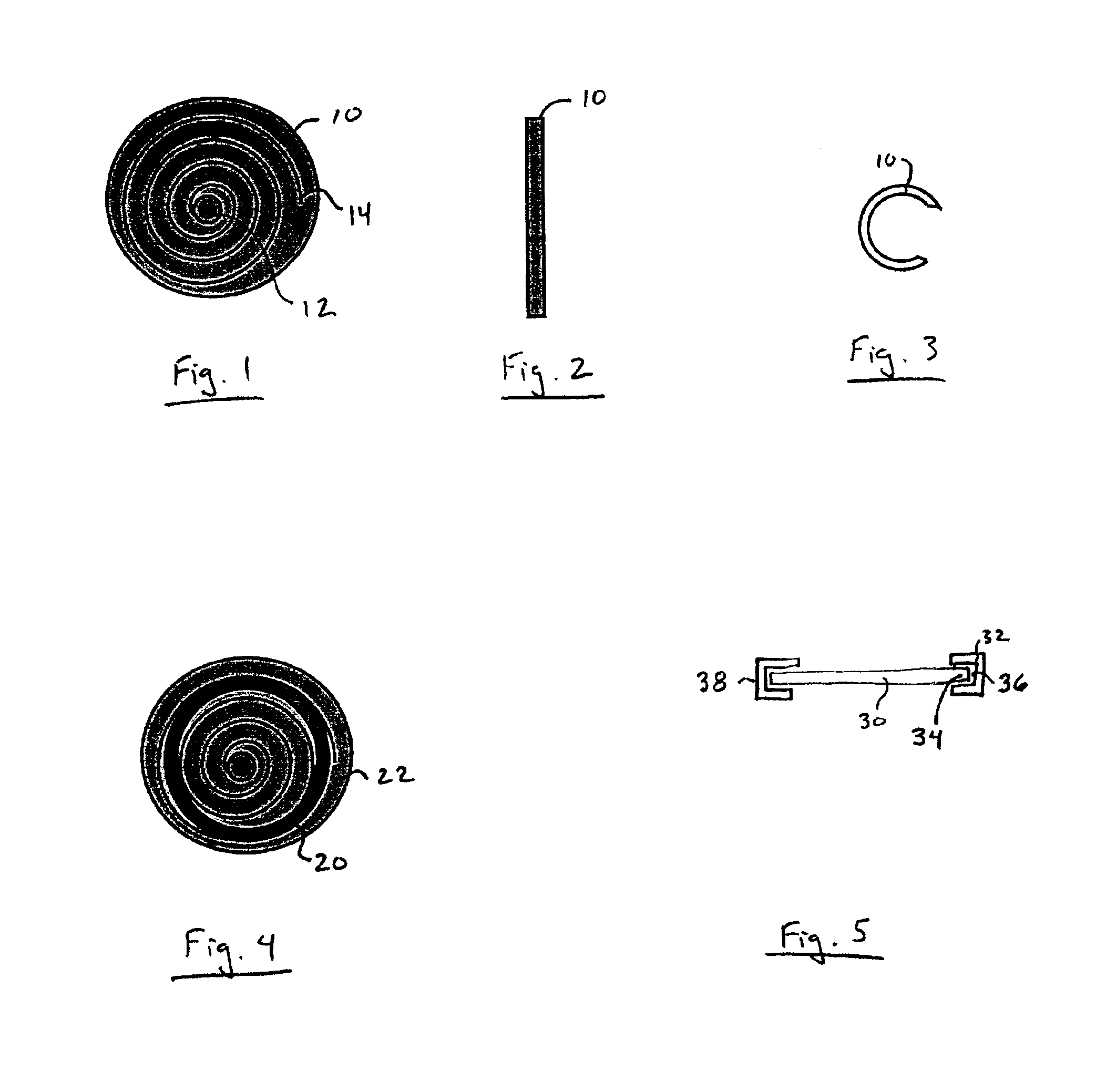
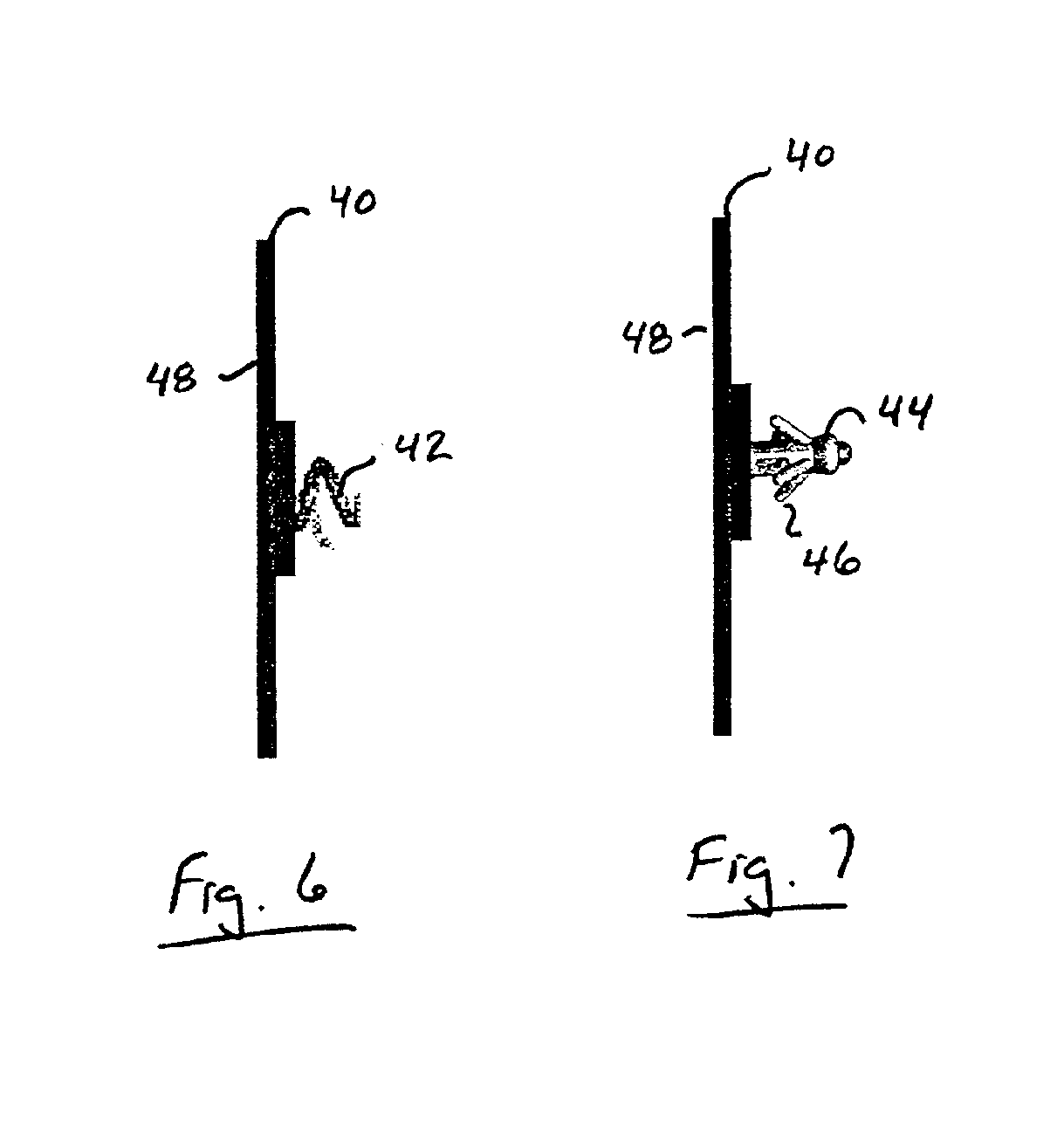
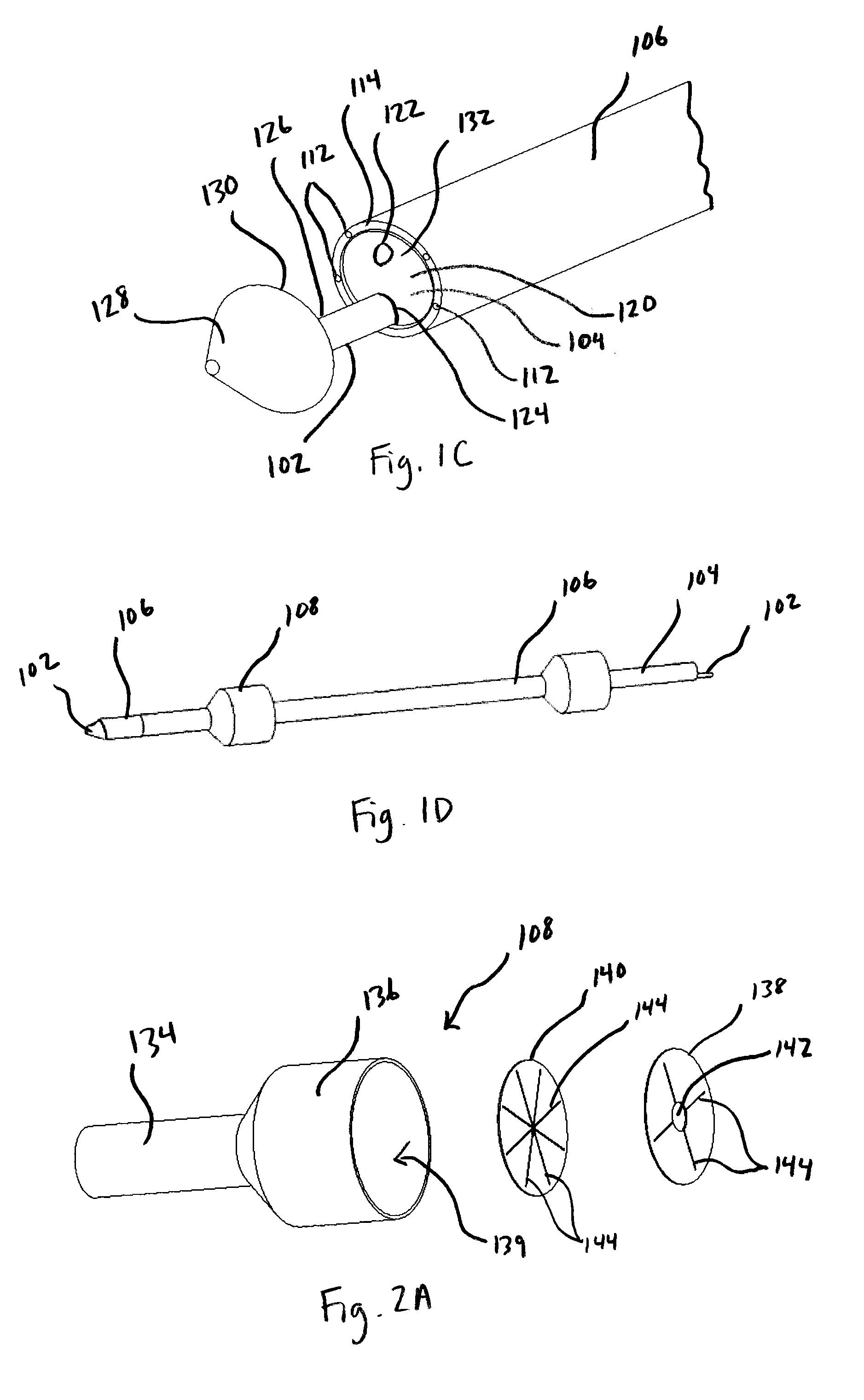
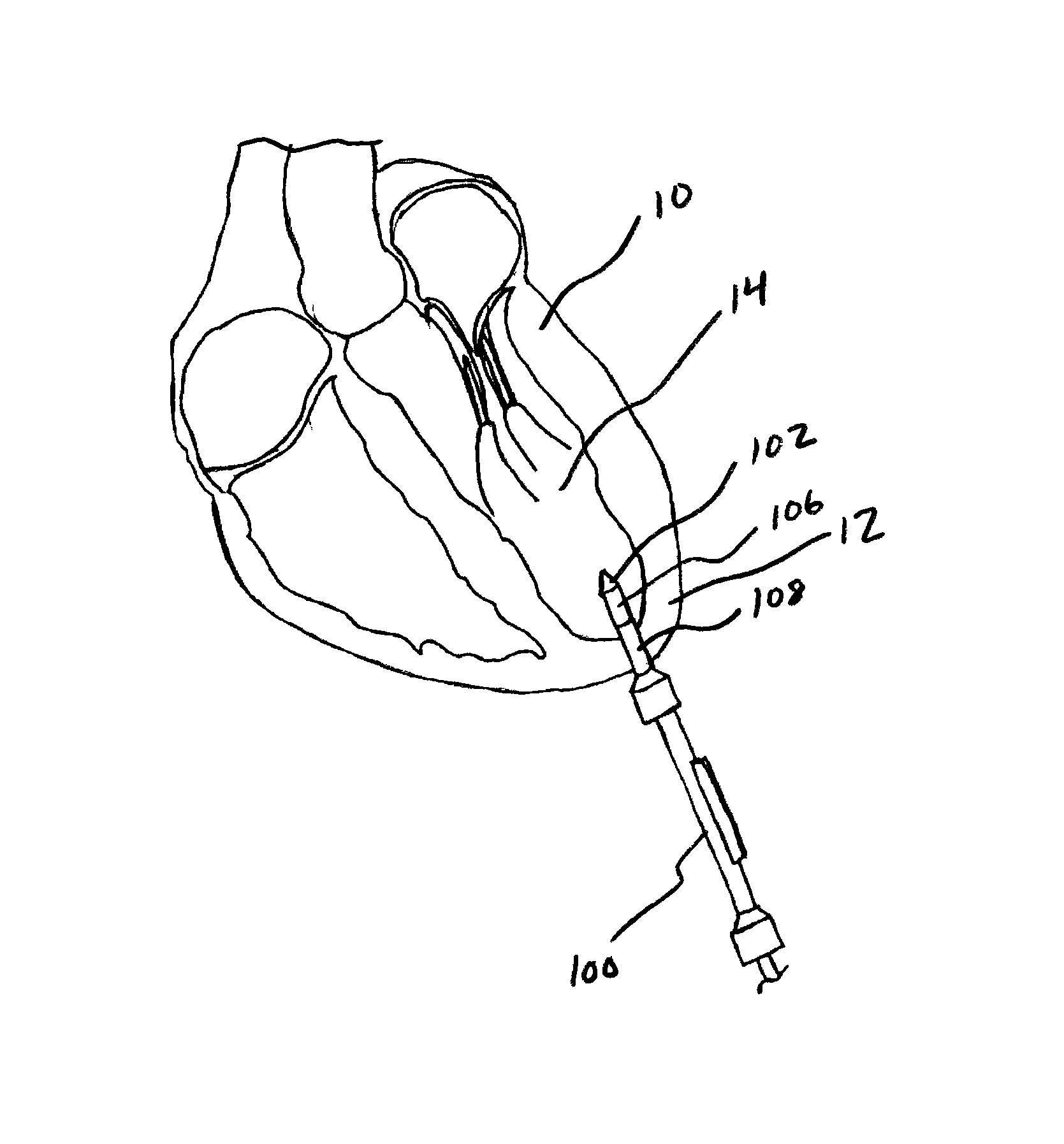
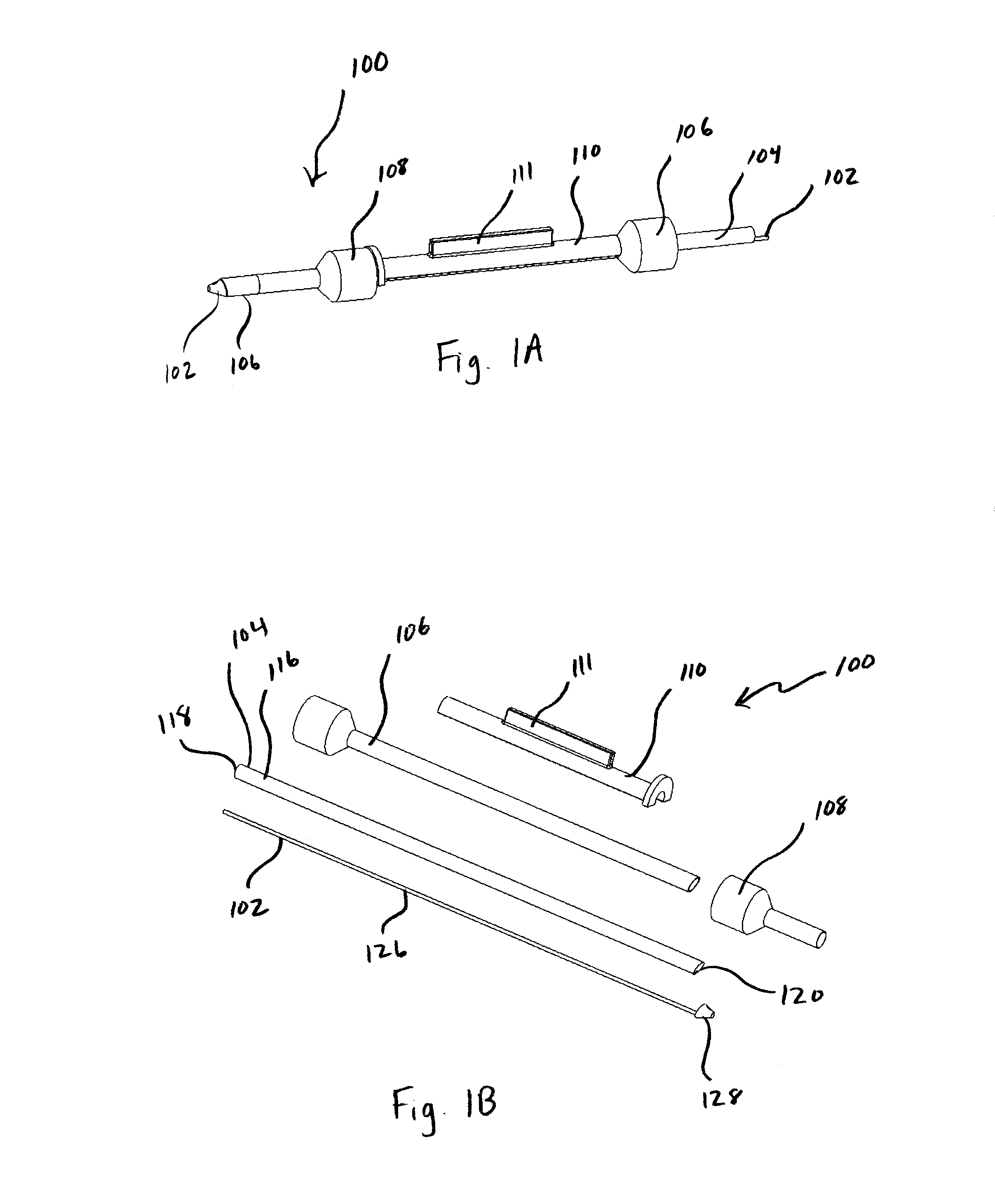
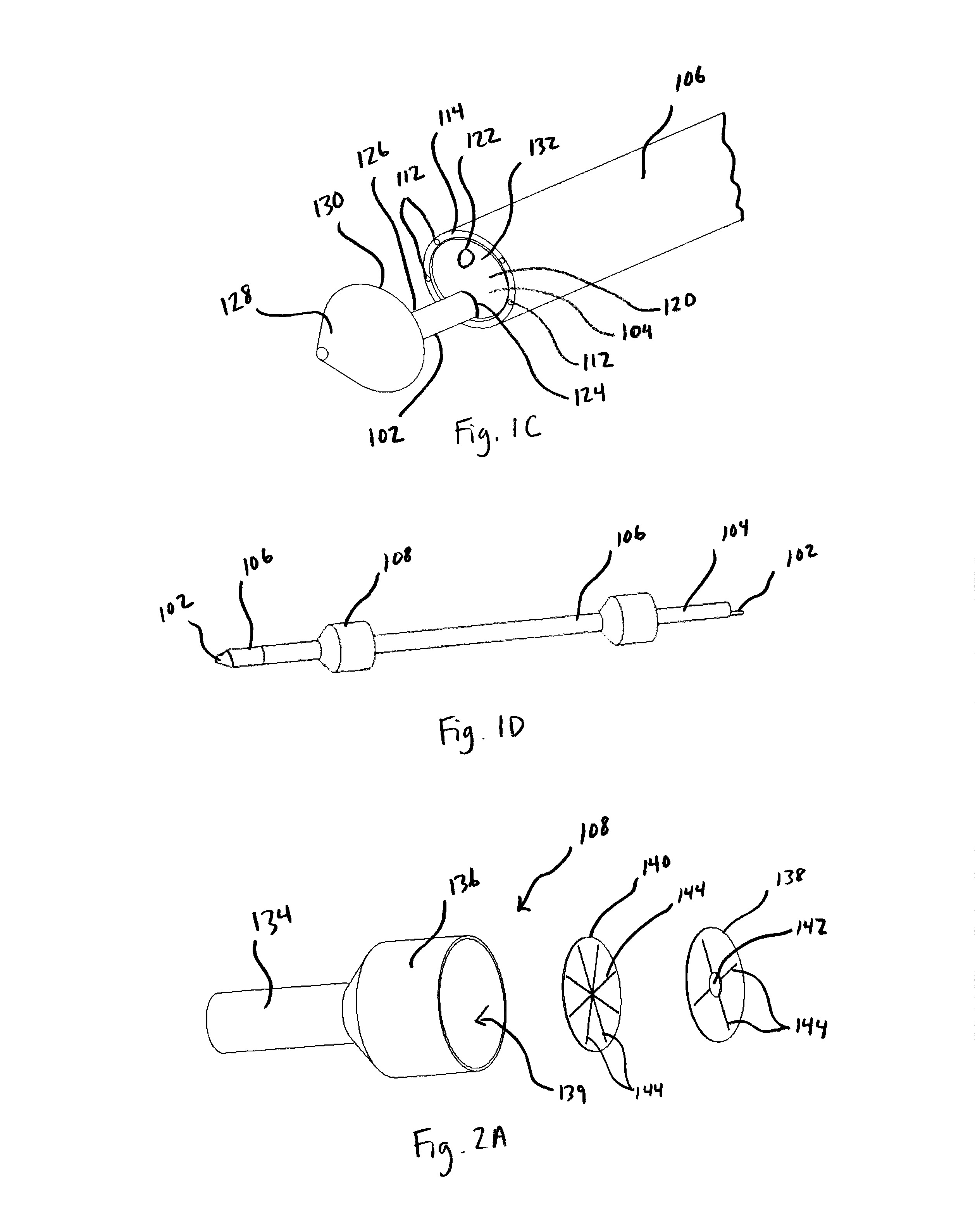
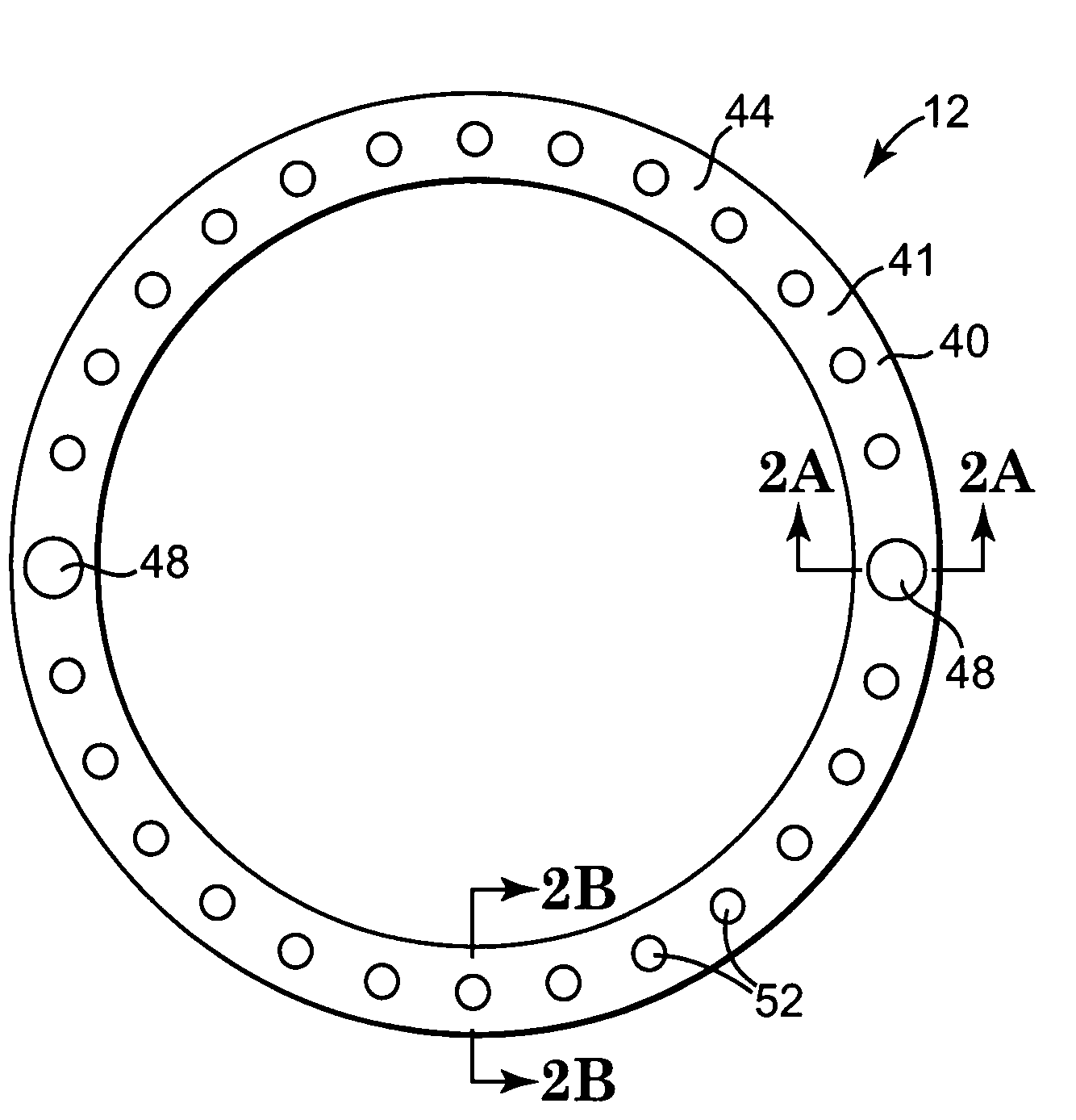
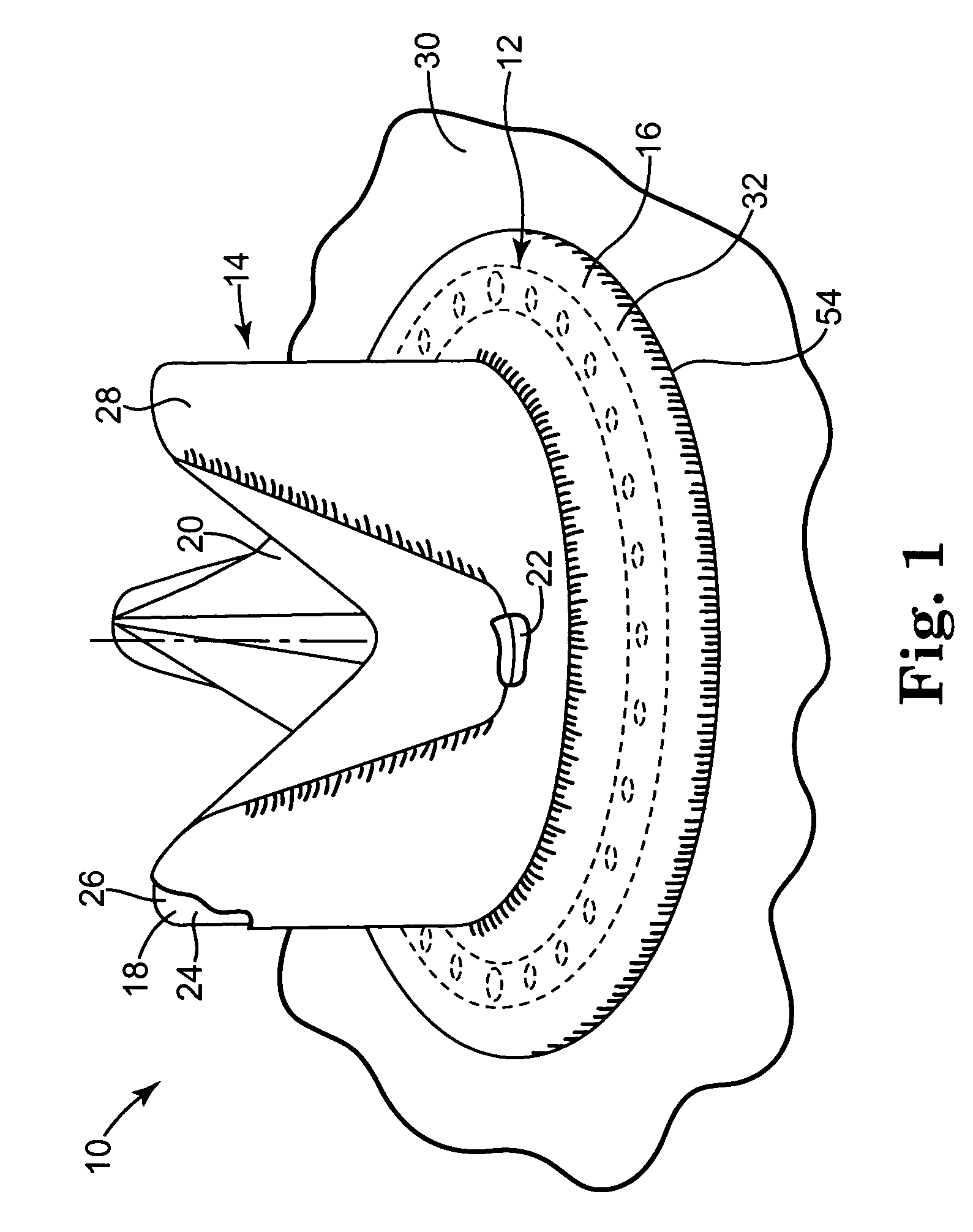
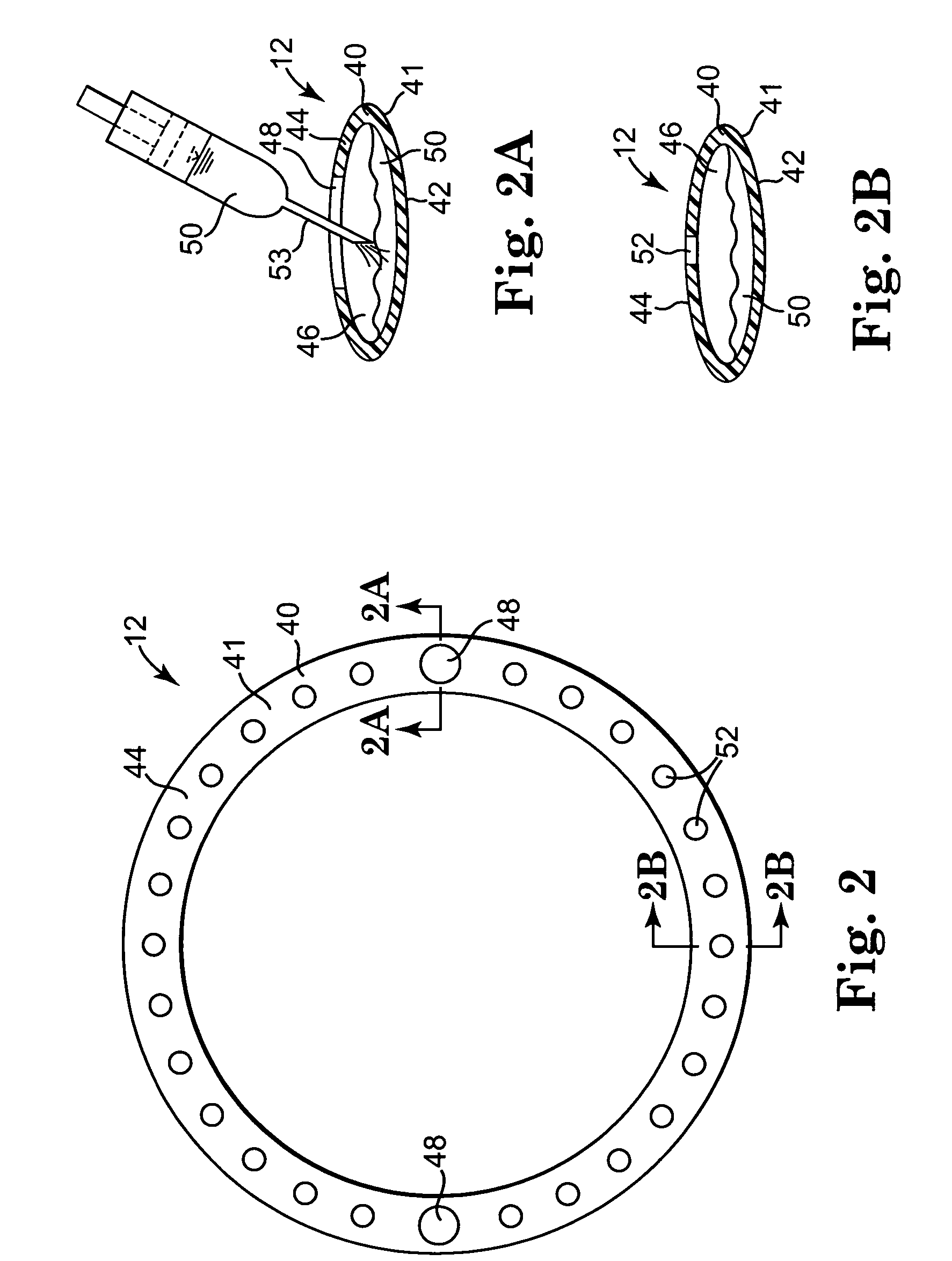
Implantable wireless sensor for pressure measurement within the heart
InactiveUS6855115B2Measure the pressure easily, safely, inexpensively and accuratelyElectrotherapyPerson identificationLine sensorHeart chamber
The progress of a endovascular cardiac repair can be monitored by inserting a pressure transducer sensor using a catheter into a chamber of the heart during endovascular repair and then using a small, hand-held read out device to measure pressure easily, safely, inexpensively and accurately. In one aspect a sensor is introduced into the body by the steps of folding or rolling the sensor into a cylinder, loading it into a catheter, and deploying into the heart chamber by allowing it to unroll or unfold, either by itself or facilitated by the incorporation of a super-elastic alloy component.
Owner:ST JUDE MEDICAL LUXEMBOURG HLDG II S A R L SJM LUX II
Pharmacological delivery implement for use with cardiac repair devices
A pharmacological delivery implement for use with cardiac repair devices. The pharmacological delivery implement comprises a porous member defining an outer surface, an internal channel configured to selectively house a pharmacological agent, and a plurality of release holes each extending from the outer surface to the internal channel. Following implantation of the pharmacological delivery implement, at least a portion of the pharmacological agent exits the internal channel through the plurality of release holes.
Owner:MEDTRONIC INC
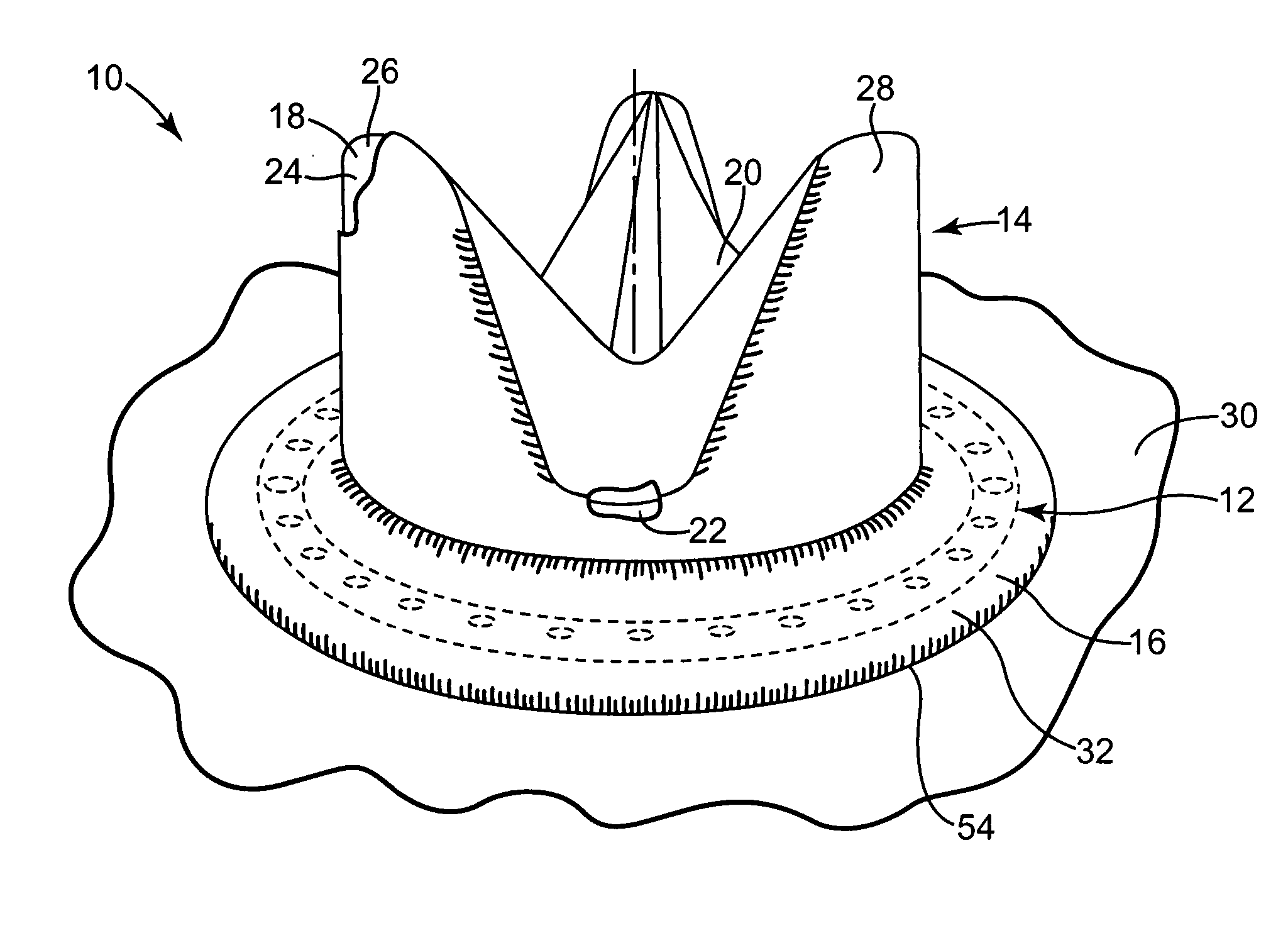
Exchangeable system for minimally invasive beating heart repair of heart valve leaflets
ActiveUS20120184971A1Prevent movementPrevent escapeSuture equipmentsCannulasHeart valve repairImproved method
Improved methods and apparatuses for heart valve repair in a beating heart of a patient utilize an exchangeable heart valve repair system. Heart valve repair system can include a port adapted to be seated in the heart wall and an imaging catheter slidable within the port. The imaging catheter can be selectively locked relative to the port for insertion into the heart and unlocked once the port is seated to allow the imaging catheter to move distally towards target tissue. A deployment catheter slidably disposed in the imaging catheter and a repair cartridge slidably disposed in the deployment catheter can be used to capture the target tissue and deploy a repair device into the tissue after proper capture is confirmed. System elements can be selectively removed from and replaced within port to deploy additional repair devices while port maintains a seal while elements are and are not inserted.
Owner:NEOCHORD
Exchangeable system for minimally invasive beating heart repair of heart valve leaflets
Improved methods and apparatuses for heart valve repair in a beating heart of a patient utilize an exchangeable heart valve repair system. Heart valve repair system can include a port adapted to be seated in the heart wall and an imaging catheter slidable within the port. The imaging catheter can be selectively locked relative to the port for insertion into the heart and unlocked once the port is seated to allow the imaging catheter to move distally towards target tissue. A deployment catheter slidably disposed in the imaging catheter and a repair cartridge slidably disposed in the deployment catheter can be used to capture the target tissue and deploy a repair device into the tissue after proper capture is confirmed. System elements can be selectively removed from and replaced within port to deploy additional repair devices while port maintains a seal while elements are and are not inserted.
Owner:NEOCHORD
Pharmacological delivery implement for use with cardiac repair devices
A pharmacological delivery implement for use with cardiac repair devices. The pharmacological delivery implement comprises a porous member defining an outer surface, an internal channel configured to selectively house a pharmacological agent, and a plurality of release holes each extending from the outer surface to the internal channel. Following implantation of the pharmacological delivery implement, at least a portion of the pharmacological agent exits the internal channel through the plurality of release holes.
Owner:MEDTRONIC INC
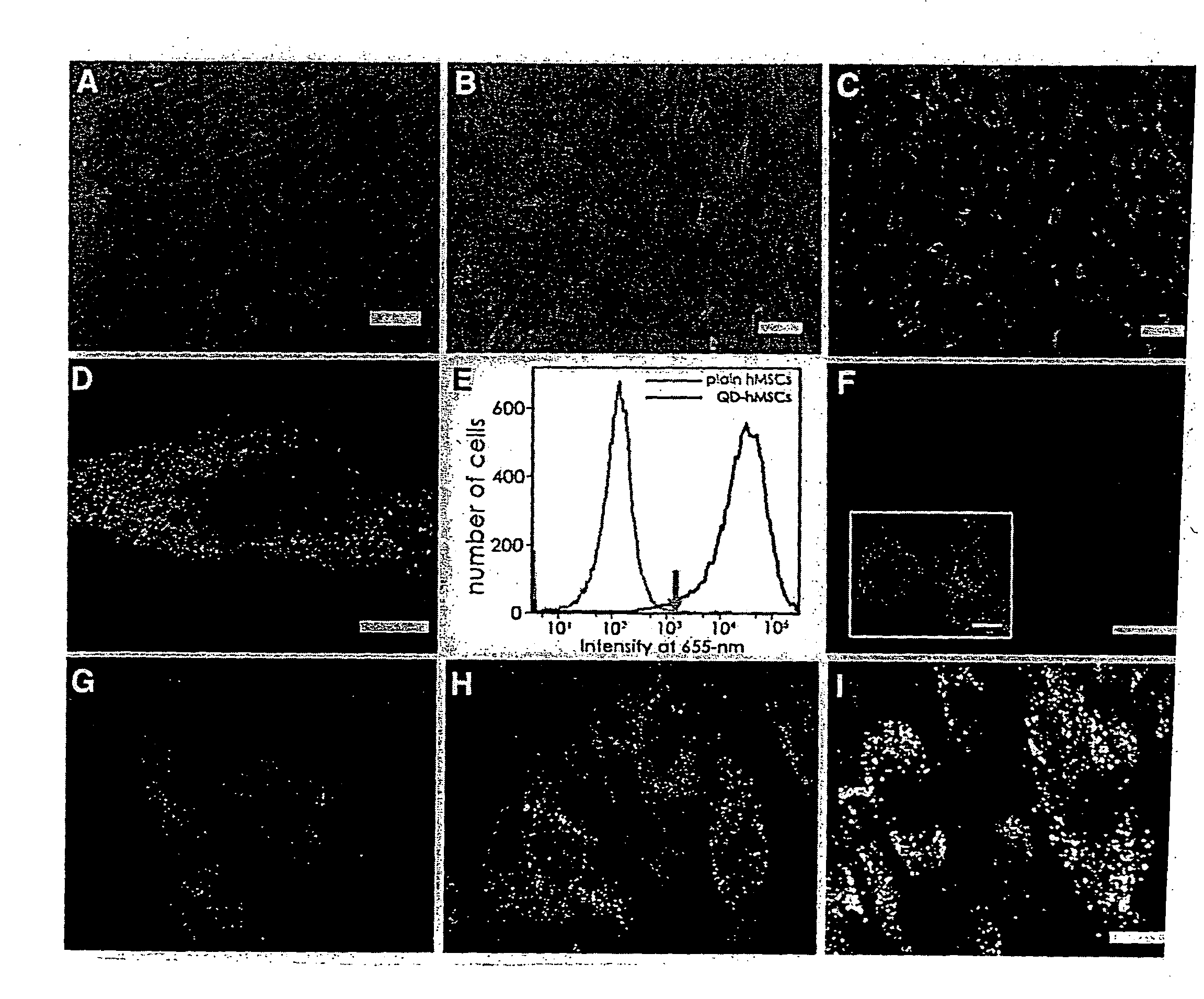
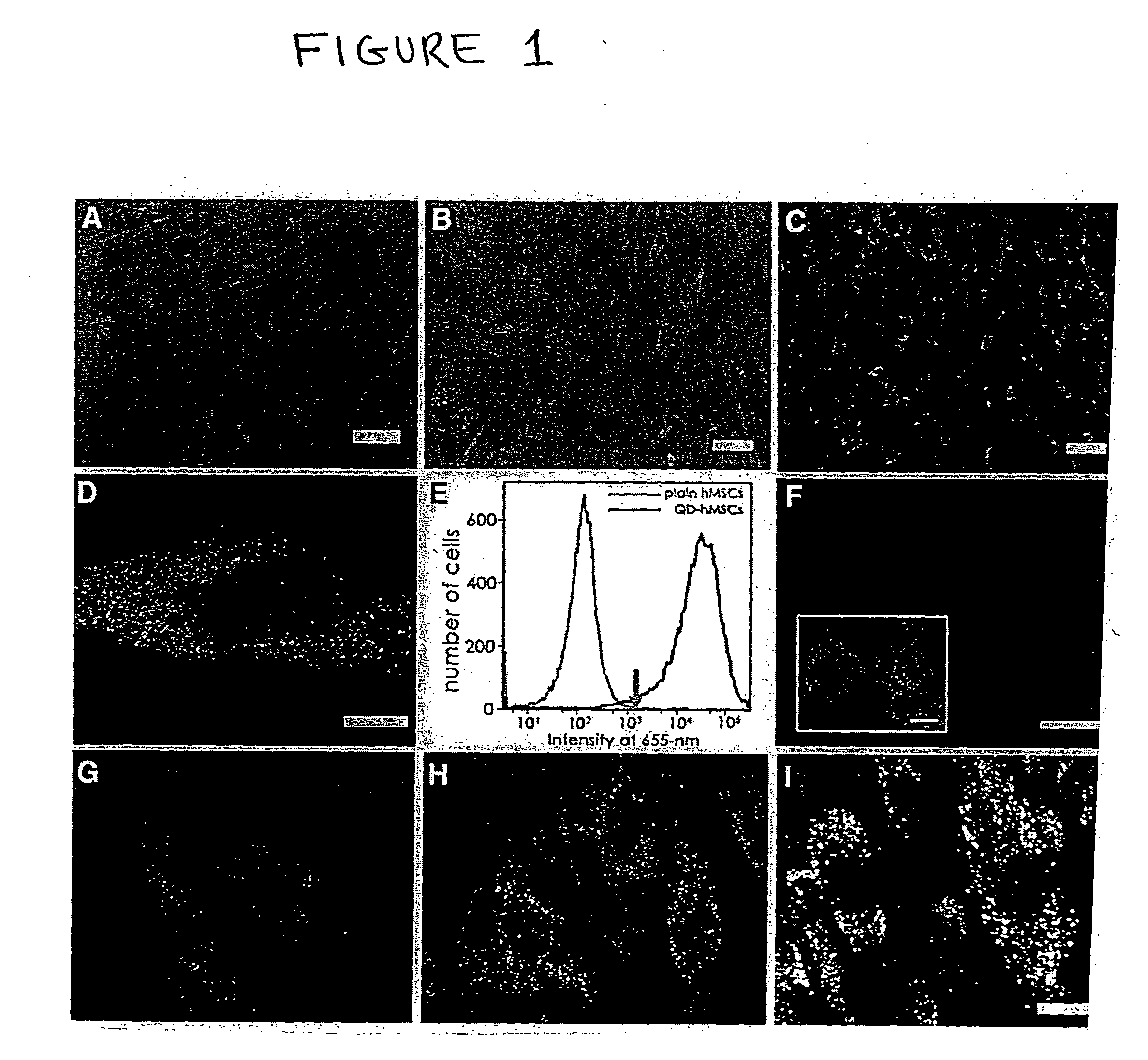
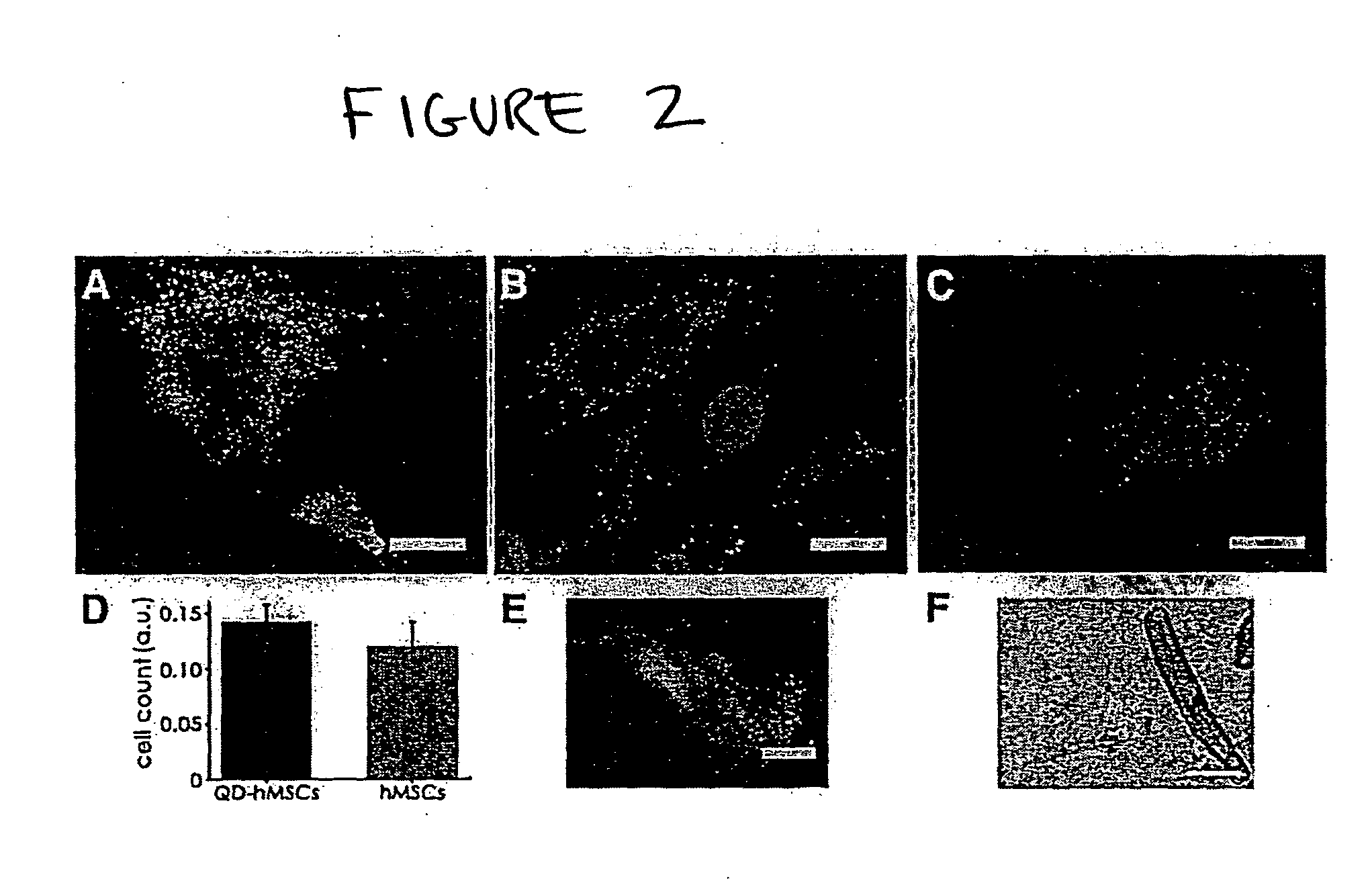
Quantum dot labeled stem cells for use in cardiac repair
InactiveUS20100158805A1Easy to useLack of cellular toxicityBiocidePowder deliveryCytosolFluorescence
The present invention provides methods and compositions relating to the labeling of target cells with quantum dots (QDs). Specifically, a delivery system is disclosed based on the use of negatively charged QDs for delivery of a tracking fluorescent signal into the cytosol of target cells via a passive endocytosis-mediated delivery process. In a specific embodiment of the invention the target cell is a stem cell, preferably a mesenchymal stem cell (MSC). Such labeled MSCs provide a means for tracking the distribution and fate of MSCs that have been administered to a subject to promote cardiac repair. The invention is based on the discovery that MSCs can be tracked in vitro for up to at least 6 weeks. Additionally, QDs delivered in vivo can be tracked for up to at least 8 weeks, thereby permitting for the first time, the complete 3-D reconstruction of the locations of all MSCs following administration into a host.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Cardiac repair, resizing and reshaping using the venous system of the heart
ActiveUS20080269720A1ThickenReducing systolic volumeSuture equipmentsHeart valvesTreatment effectCardiac functioning
Structurally supportive material is implanted or injected into cardiac veins as discrete masses at various sites in the cardiac venous system to reinforce the myocardium for the purpose of preventing, moderating, stopping or reversing negative cardiac remodeling due to various adverse cardiac conditions, both acute and chronic, or for the purpose of treating localize anomalies of the heart, or for both purposes. The sites may be arranged in a pattern about one or more chambers of the heart such that the masses cooperatively reduce stress in the chamber wall and reduce chamber size. Some patterns also cause a beneficial global reshaping of the chamber. These changes occur quickly and are sustainable, and have a rapid and sustainable therapeutic effect on cardiac function. Patterns of distribution of discrete masses in the heart for global resizing and reshaping may also be used as is or augmented by supplemental patterns to treat localized conditions such as myocardial infarctions and overt aneurysm of the ventricular wall as typically forms in response to large transmural myocardial infarctions. These techniques may also be used to treat localized conditions that may not yet have progressed to cardiomyopathy.
Owner:HENRY FORD HEALTH SYST
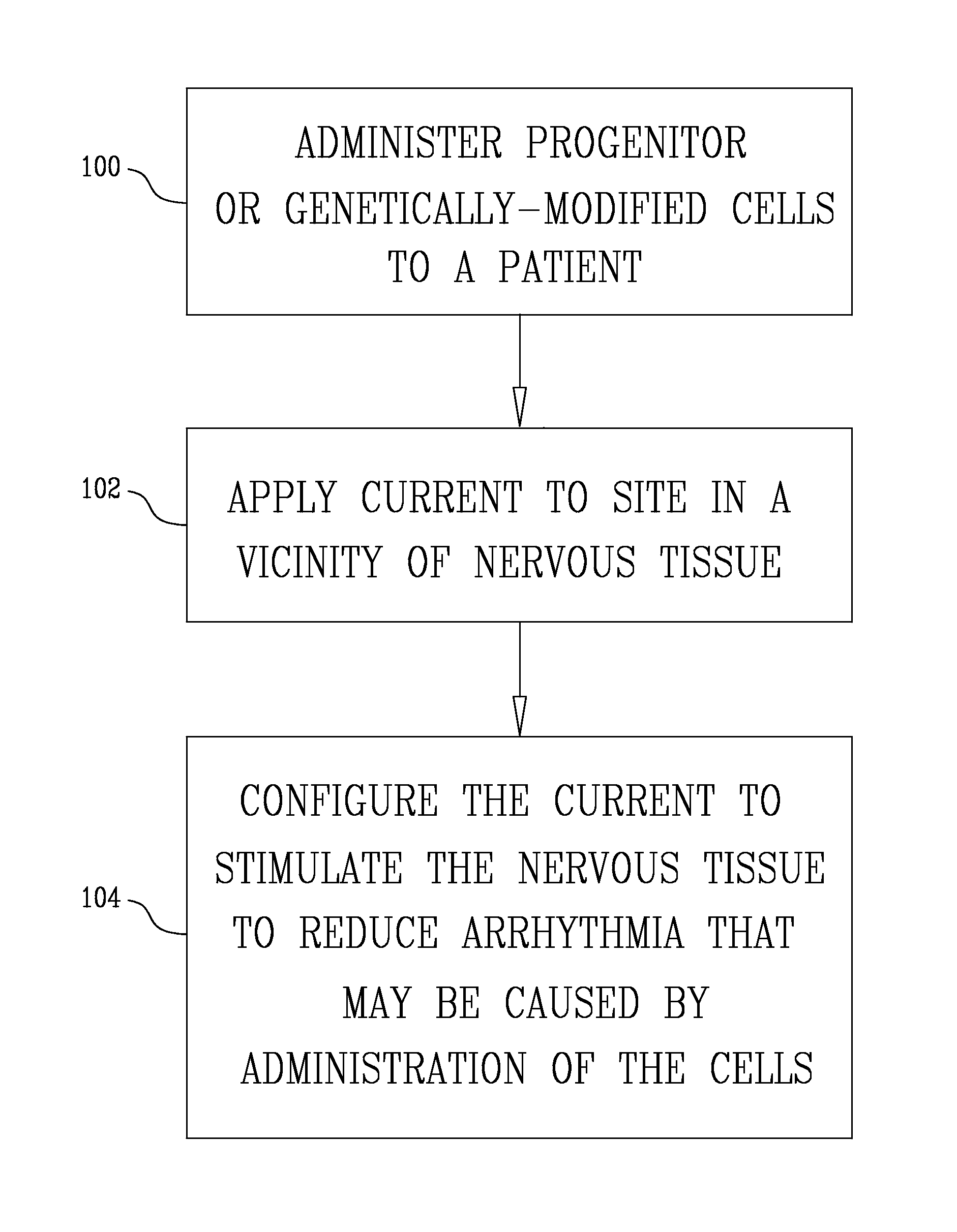
Administering bone marrow progenitor cells or myoblasts followed by application of an electrical current for cardiac repair, increasing blood supply or enhancing angiogenesis
InactiveUS8609082B2Improves the engraftment of the progenitorFunction increaseBiocideHeart stimulatorsProgenitorNeurulation
A method is provided including selecting a patient suffering from a condition, administering cells to the patient selected from the group consisting of: progenitor cells and genetically-modified cells, applying an electrical current to a site of the patient in a vicinity of nervous tissue, and configuring the current to stimulate the nervous tissue. Other embodiments are also described.
Owner:MEDTRONIC INC
Use of human stem cells and/or factors they produce to promote adult mammalian cardiac repair through cardiomyocyte cell division
A method for treating a subject afflicted with a cardiac disorder, in vivo, comprising (i) producing a solution comprising media conditioned from the culture of cells, in vitro, and (ii) administering the solution of step (i) to the subject, thereby treating the cardiac disorder in the subject. Methods for determining whether an agent stimulates or inhibits myocyte proliferation.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Differentiation of human mesenchymal stem cells to cardiac progenitor cells that promote cardiac repair
A method for treating a subject afflicted with a cardiac disorder, in vivo, comprises (i) inducing differentiation of a progenitor cell, in vitro, to a cardiogenic cell; and (ii) administering a therapeutically effective amount of the cardiogenic cell of step (i) to the subject, thereby treating the cardiac disorder in the subject. This invention further provides related articles of manufacture and methods.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Cardiac repair, resizing and reshaping using the venous system of the heart
ActiveUS7875017B2Reduce volumeFunction increaseSuture equipmentsHeart valvesDiseaseCardiac functioning
Structurally supportive material is implanted or injected into cardiac veins as discrete masses at various sites in the cardiac venous system to reinforce the myocardium for the purpose of preventing, moderating, stopping or reversing negative cardiac remodeling due to various adverse cardiac conditions, both acute and chronic, or for the purpose of treating localize anomalies of the heart, or for both purposes. The sites may be arranged in a pattern about one or more chambers of the heart such that the masses cooperatively reduce stress in the chamber wall and reduce chamber size. Some patterns also cause a beneficial global reshaping of the chamber. These changes occur quickly and are sustainable, and have a rapid and sustainable therapeutic effect on cardiac function. Patterns of distribution of discrete masses in the heart for global resizing and reshaping may also be used as is or augmented by supplemental patterns to treat localized conditions such as myocardial infarctions and overt aneurysm of the ventricular wall as typically forms in response to large transmural myocardial infarctions. These techniques may also be used to treat localized conditions that may not yet have progressed to cardiomyopathy.
Owner:HENRY FORD HEALTH SYST
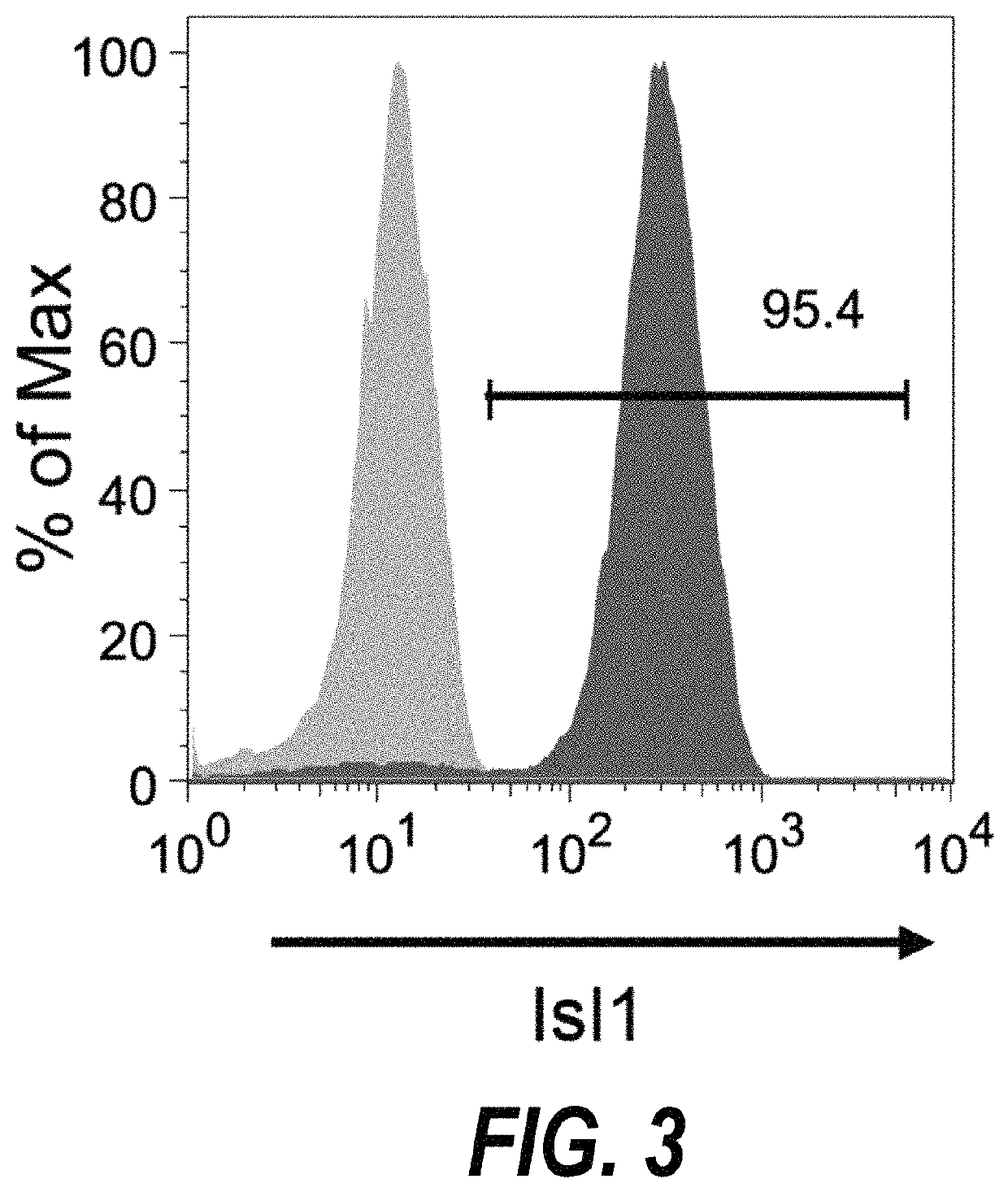
Use of islet 1 as a marker for isolating or generating stem cells
InactiveUS7745113B2Microbiological testing/measurementGenetically modified cellsProgenitorPancreatic islets
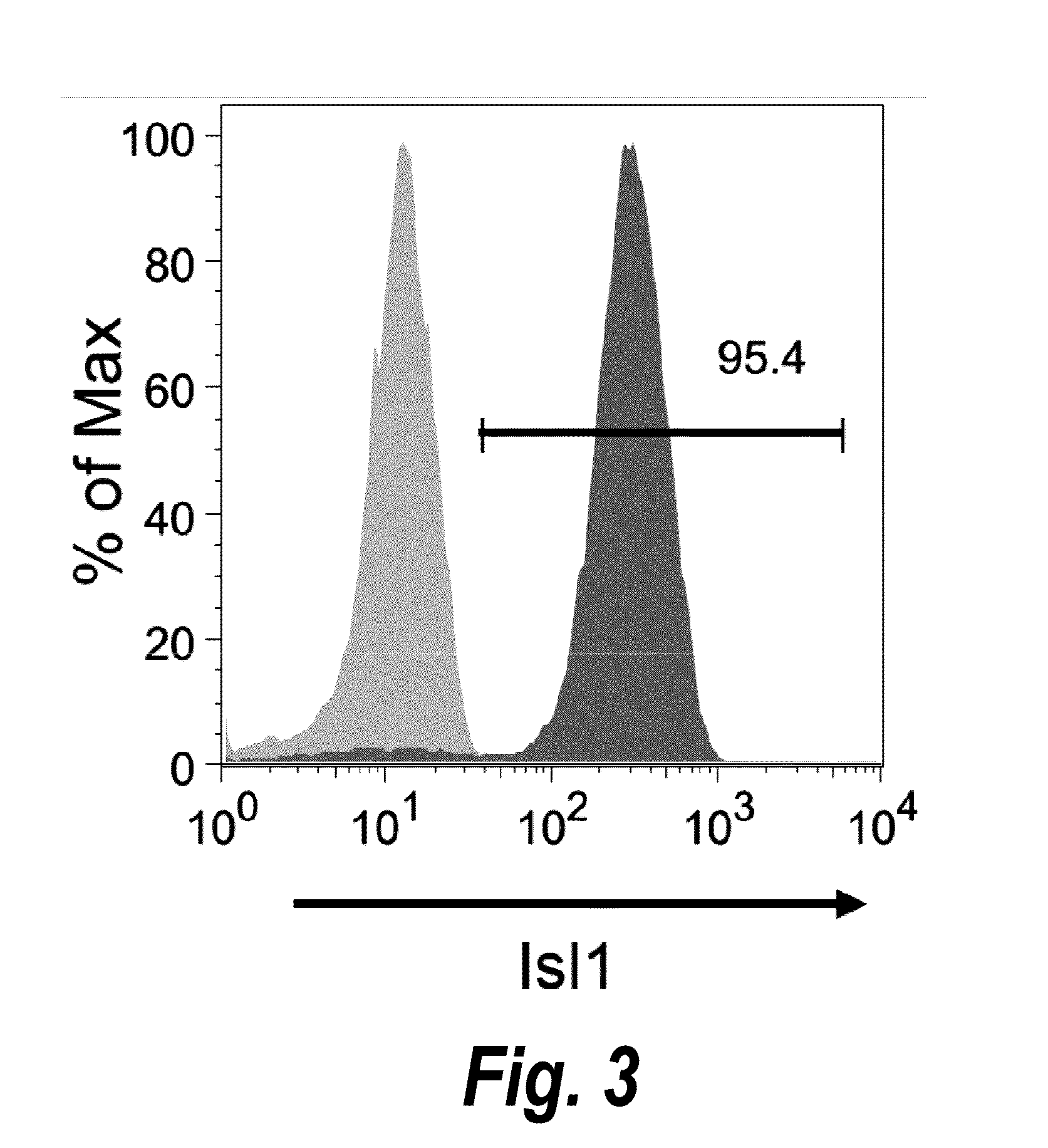
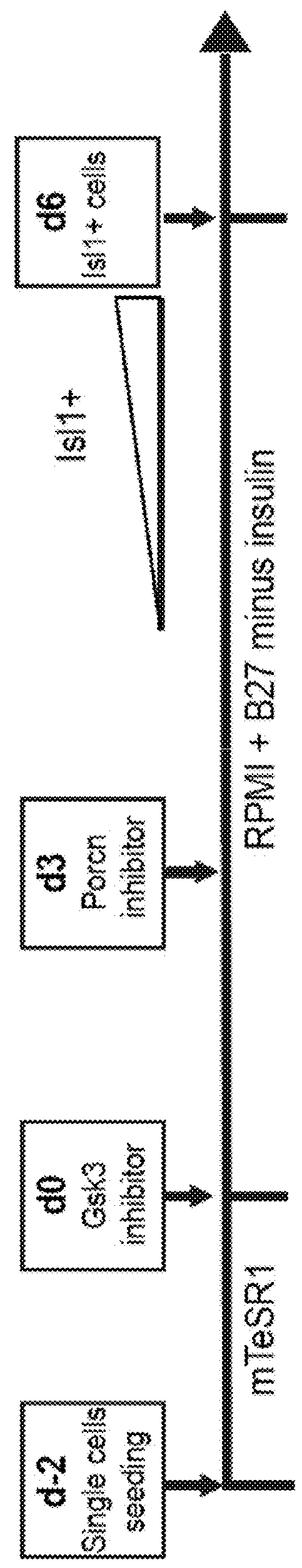
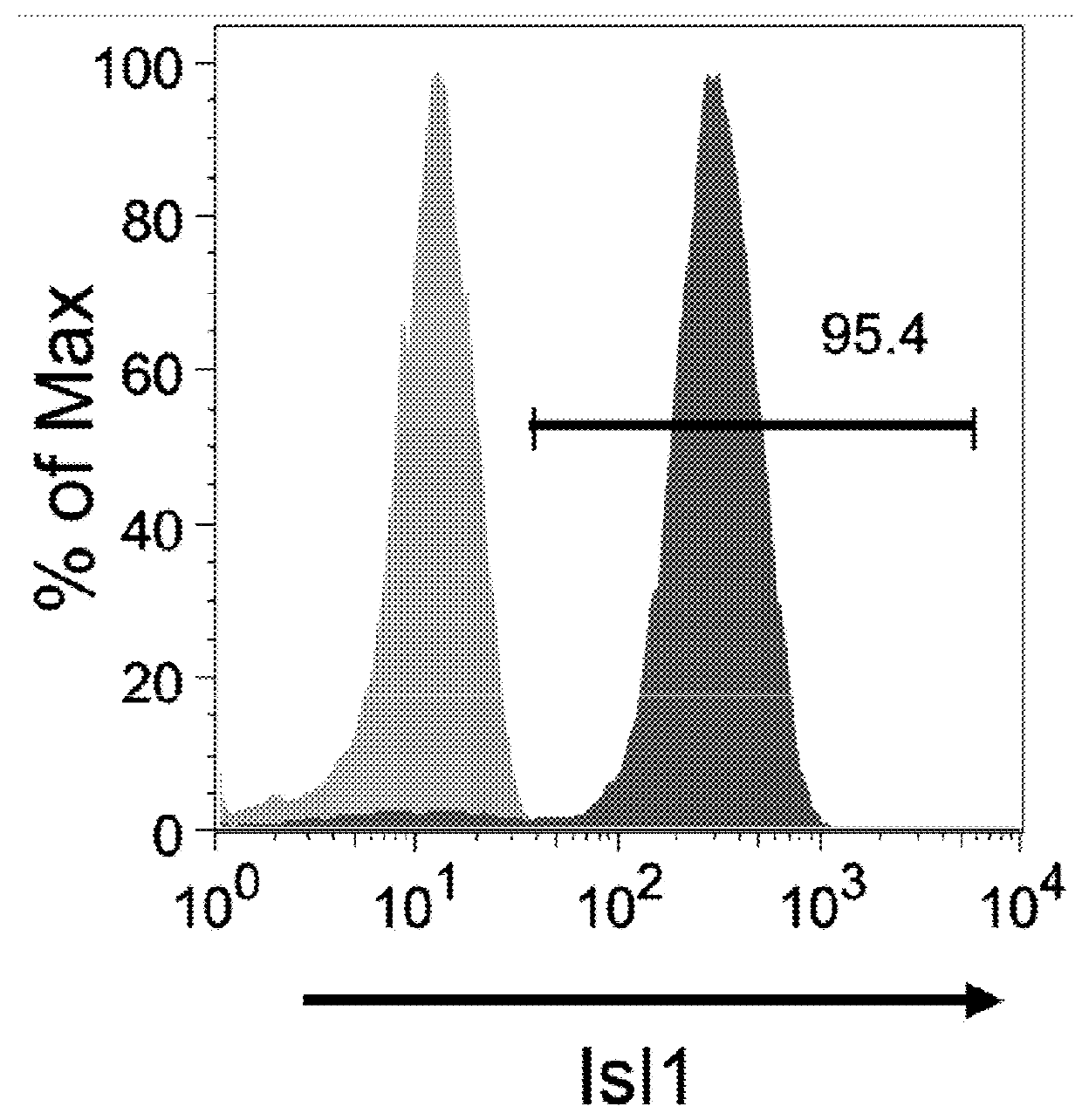
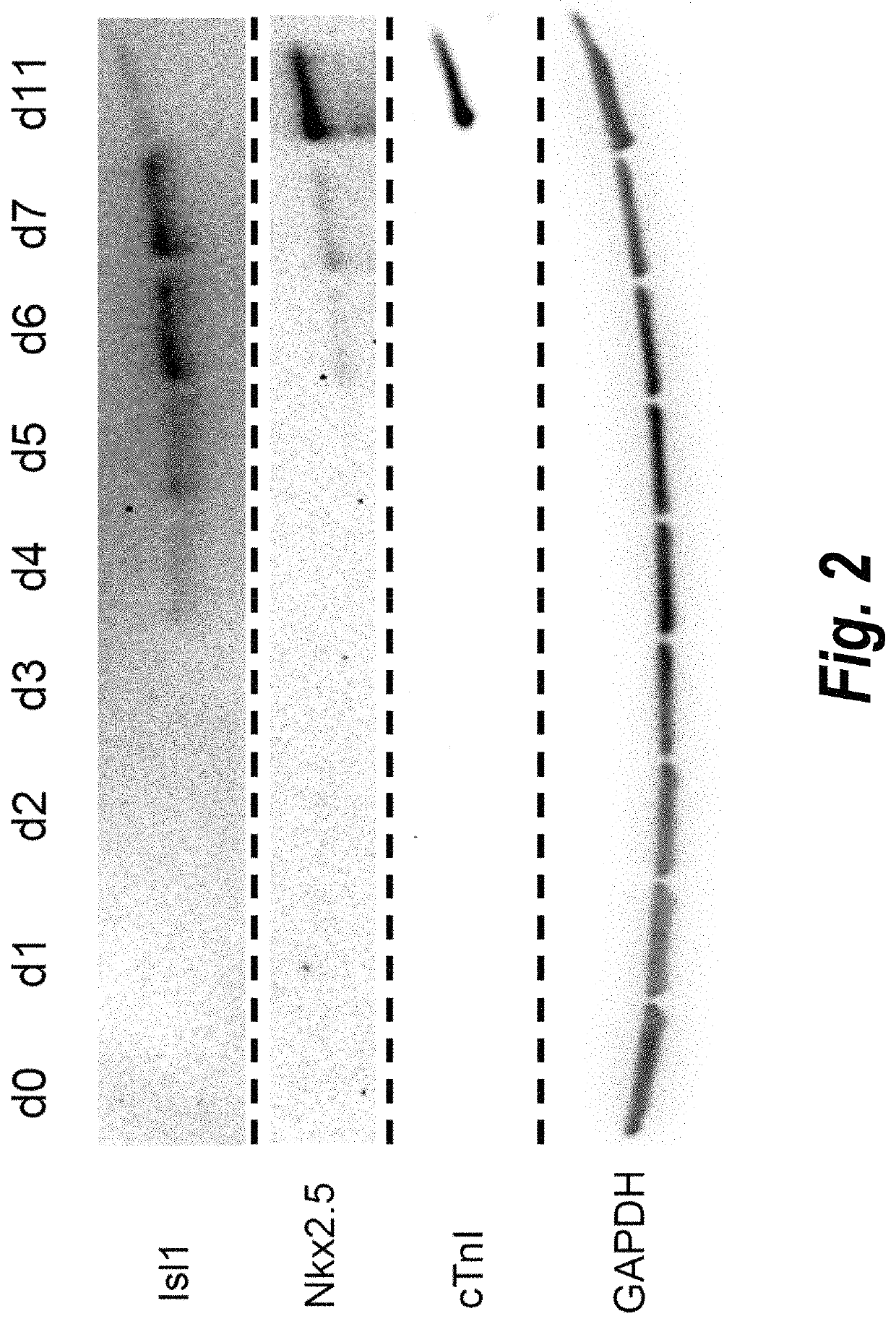
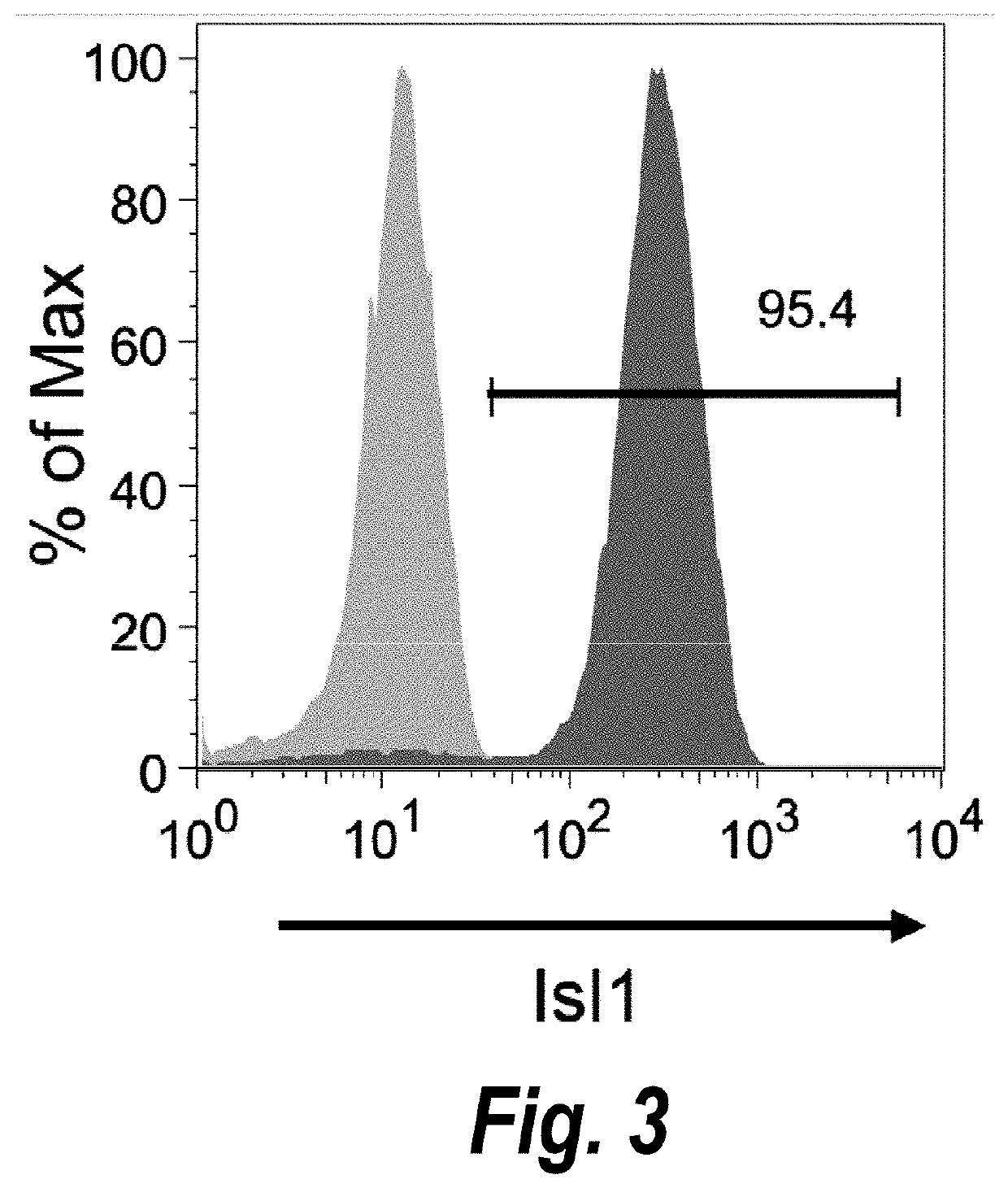
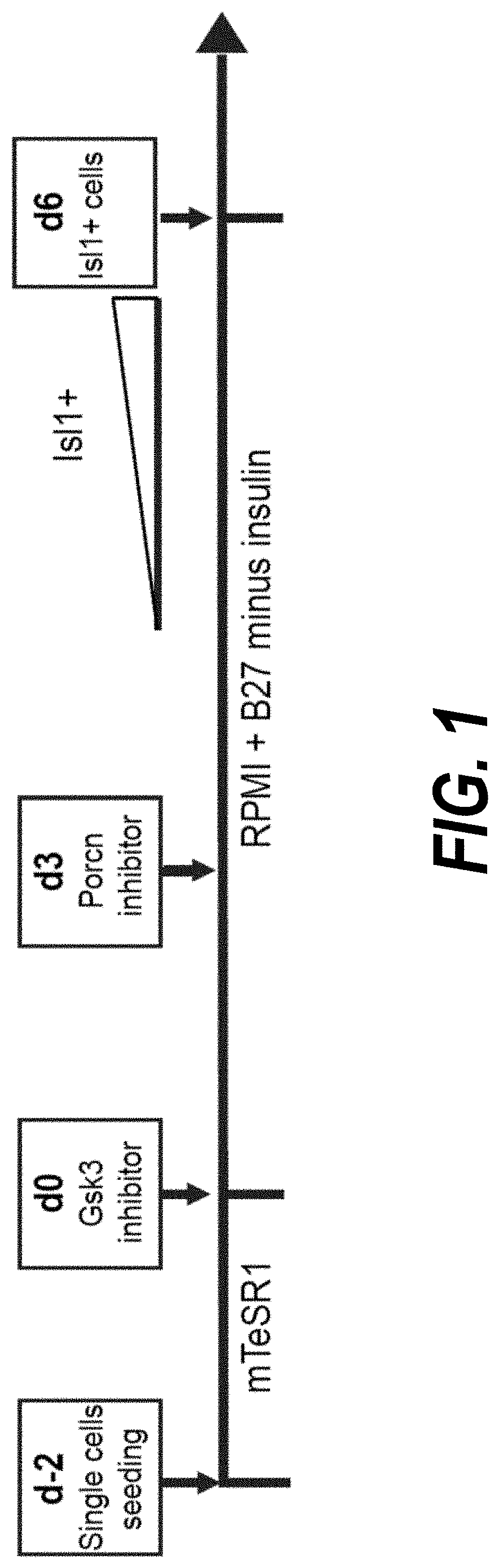
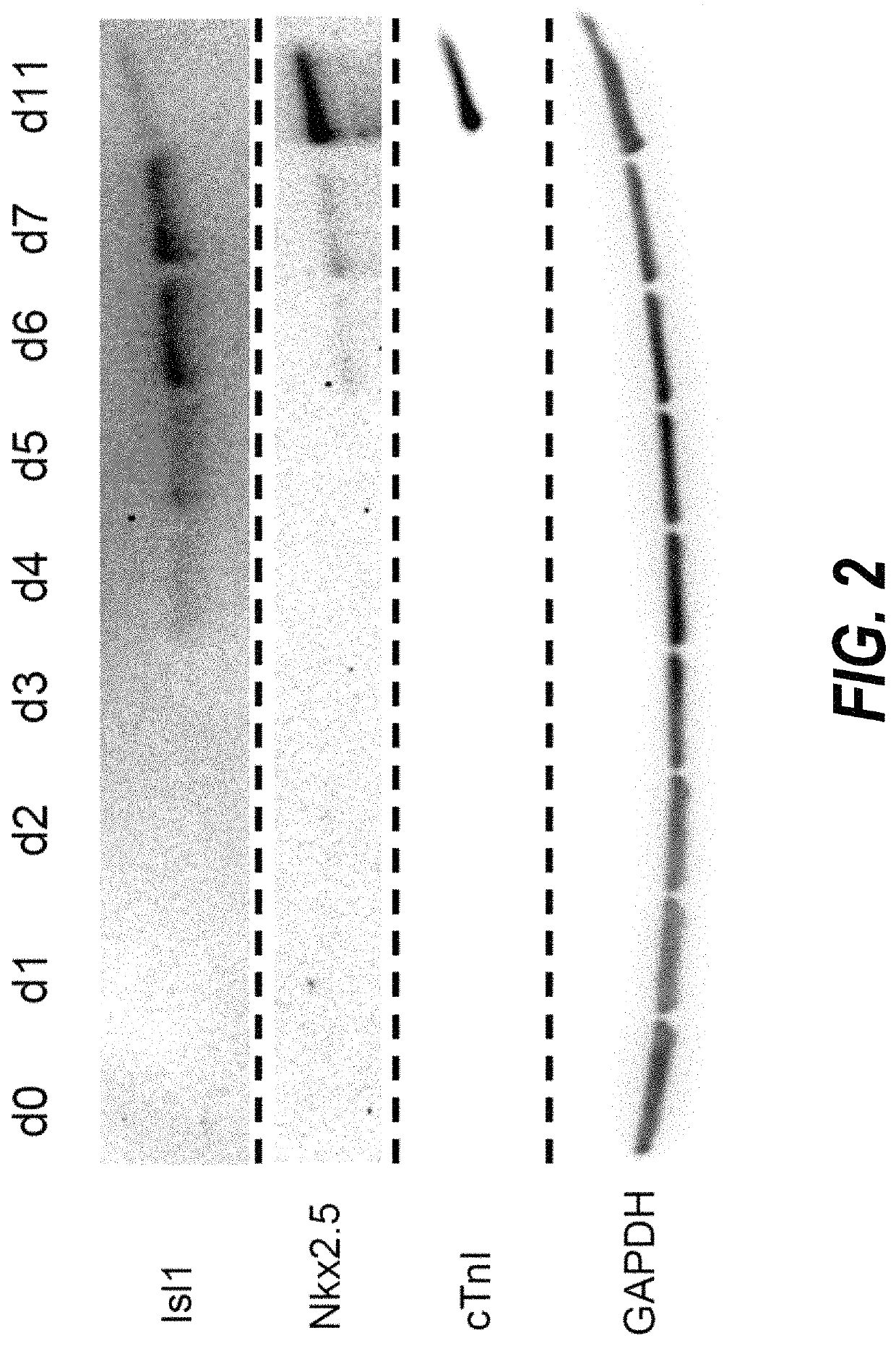
The present invention provides in vitro methods of expansion and propagation of undifferentiated progenitor cells and more specifically undifferentiated progenitor cells containing Islet1, a marker apparently unique to proliferating cardiac stem cells. Methods are described for isolation of stem cell populations as well as for provoking expansion and propagation of undifferentiated progenitor cells without differentiation, to provide cardiac repair or improve cardiac function, for example.
Owner:RGT UNIV OF CALIFORNIA
Use of lifr or fgfr3 as a cell surface marker for isolating human cardiac ventricular progenitor cells
ActiveUS20160108363A1Easy and rapid isolationFunction increaseBiocideArtificial cell constructsSurface markerProgenitor
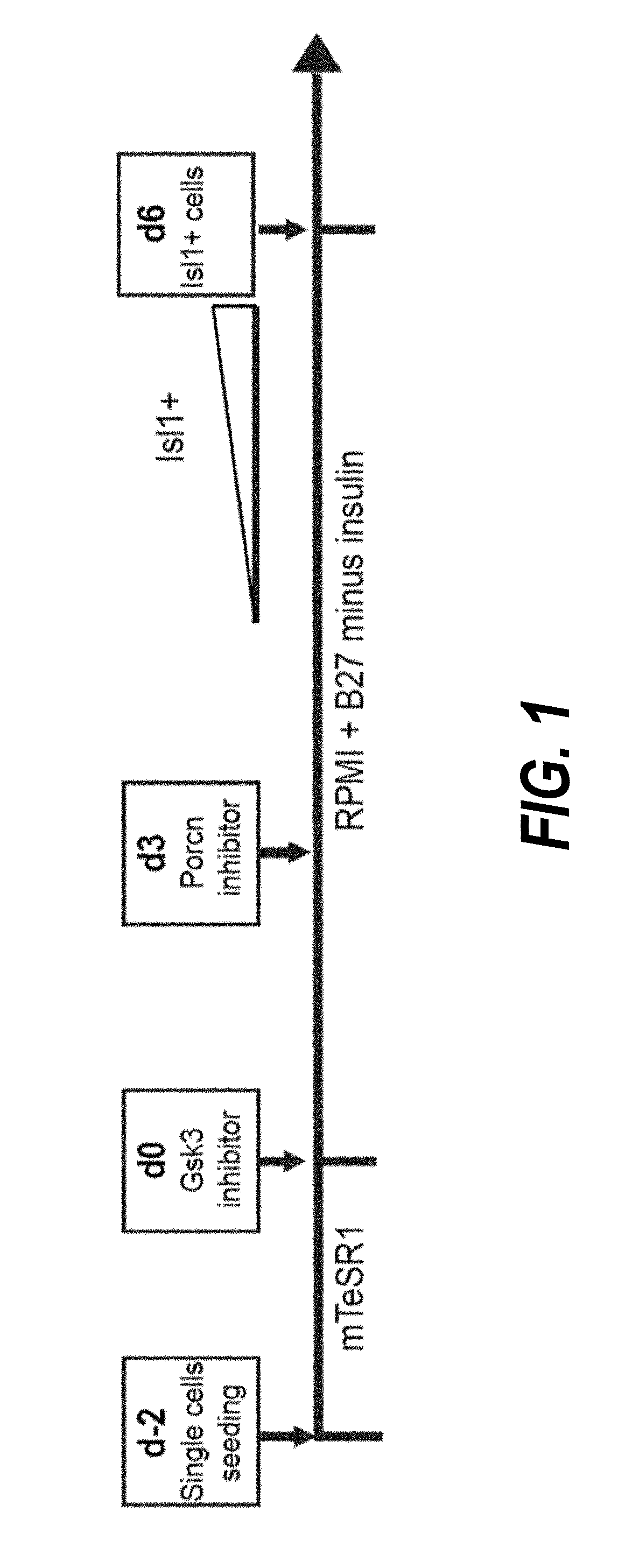
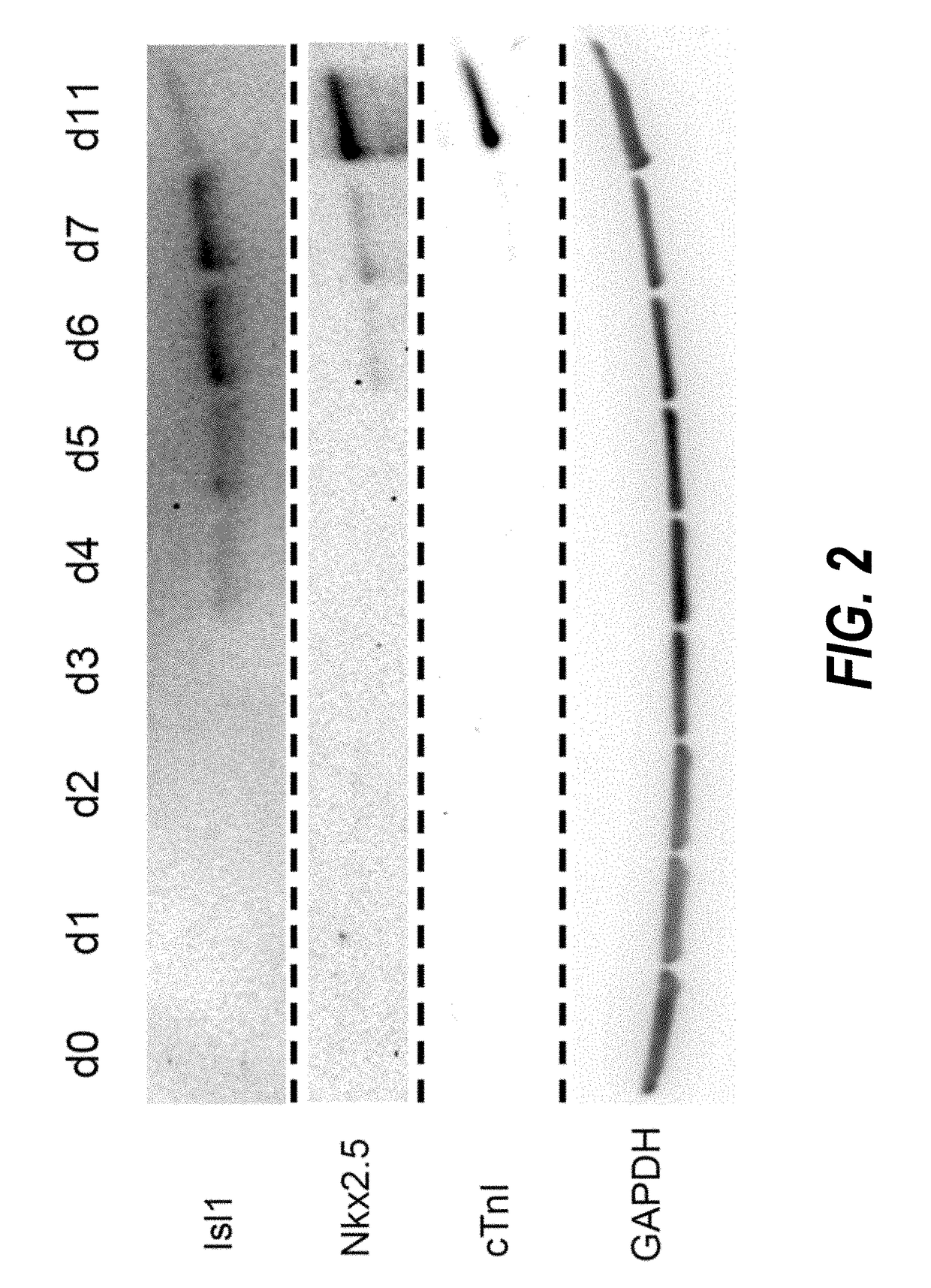
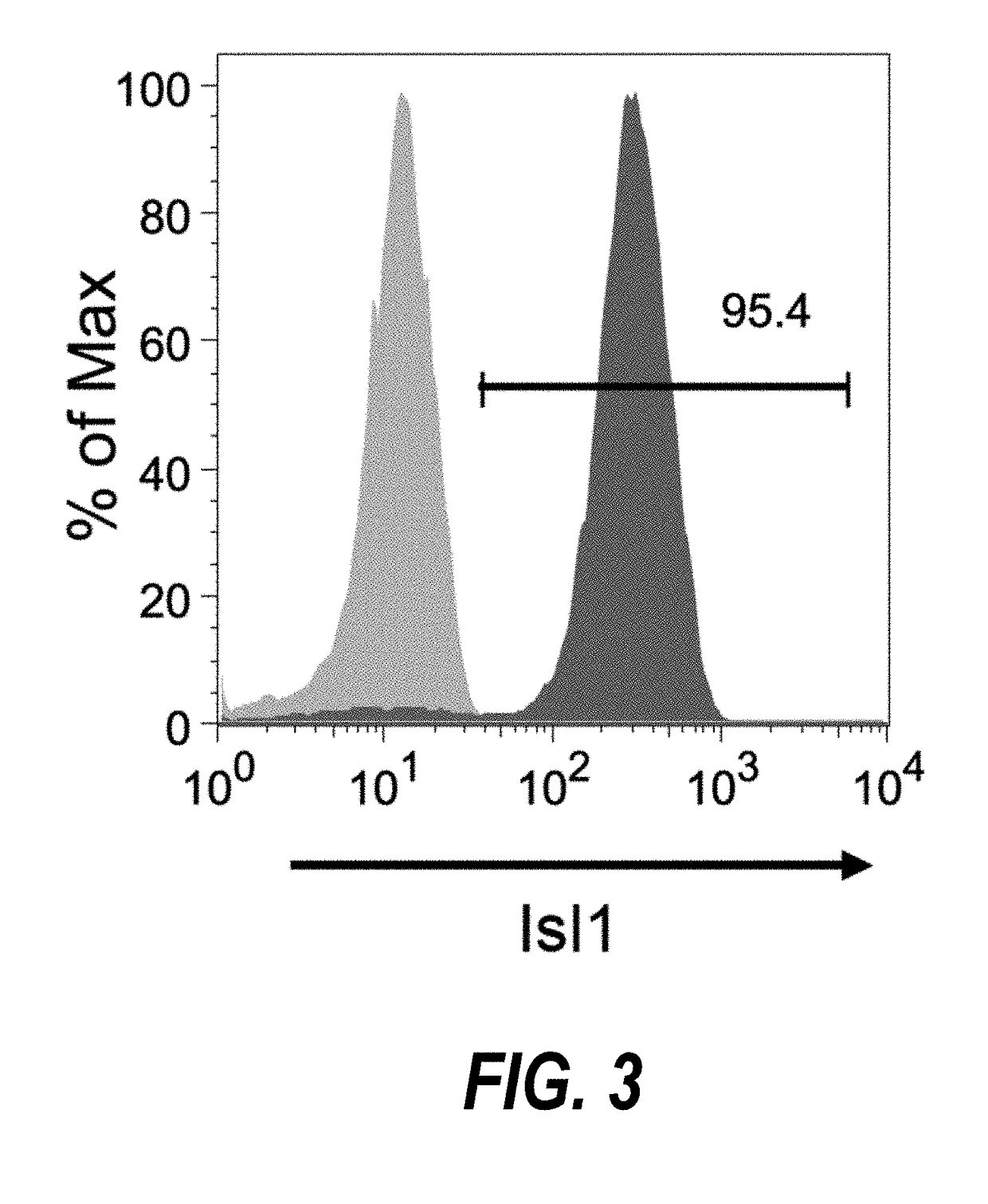
The present invention provides LIFR and FGFR3 as cell surface markers for isolating human cardiomyogenic ventricular progenitor cells, in particular progenitor cells that preferentially differentiate into cardiac ventricular muscle cells. Thus, the invention provides human ventricular progenitor (HVP) cells. The invention provides in vitro methods of the separation of Islet 1+ LIFR+ ventricular progenitor cells and / or Islet 1+ / FGFR3+ ventricular progenitor cells and / or Islet 1+ / LIFR+ / FGFR3+ ventricular progenitor cells, and the large scale expansion and propagation thereof. Large clonal populations of isolated LIFR+ and / or FGFR3+ ventricular progenitor cells are also provided. Methods of in vivo use of LIFR+ and / or FGFR3+ ventricular progenitor cells for cardiac repair or to improve cardiac function are also provided. Methods of using the LIFR+ and / or FGFR3+ ventricular progenitor cells for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
Use of jagged 1/frizzled 4 as a cell surface marker for isolating human cardiac ventricular progenitor cells
ActiveUS20160053229A1Easy and rapid isolationFunction increaseBiocideDrug screeningSurface markerProgenitor
The present invention provides Jagged 1 and Frizzled 4 as cell surface markers for isolating human cardiomyogenic ventricular progenitor cells, in particular progenitor cells that preferentially differentiate into cardiac ventricular muscle cells. Thus, the invention provides human ventricular progenitor (HVP) cells. The invention provides in vitro methods of the separation of Islet 1+ Jagged 1+ ventricular progenitor cells and / or Islet 1+ / Frizzled 4+ ventricular progenitor cells and / or Islet 1+ / Jagged 1+ / Frizzled 4+ ventricular progenitor cells, and the large scale expansion and propagation thereof. Large clonal populations of isolated Jagged 1+ and / or Frizzled 4+ventricular progenitor cells are also provided. Methods of in vivo use of Jagged 1+ and / or Frizzled 4+ ventricular progenitor cells for cardiac repair or to improve cardiac function are also provided. Methods of using the Jagged 1+ and / or Frizzled 4+ ventricular progenitor cells for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
Use of neuropilin-1 (NRP1) as a cell surface marker for isolating human cardiac ventricular progenitor cells
ActiveUS20190062696A1Easy and rapid isolationFunction increaseCulture processCell culture supports/coatingProgenitorSurface marker
The present invention provides NRP1 as a cell surface marker for isolating human cardiomyogenic ventricular progenitor cells (HVPs), in particular progenitor cells that preferentially differentiate into cardiac ventricular muscle cells. Additional HVP cell surface markers identified by single cell sequencing are also provided. The invention provides in vitro methods of the separation of NRP1+ ventricular progenitor cells, and the large scale expansion and propagation thereof. Large clonal populations of isolated NRP1+ ventricular progenitor cells are also provided. Methods of in vivo use of NRP1+ ventricular progenitor cells for cardiac repair or to improve cardiac function are also provided. Methods of using the NRP1+ ventricular progenitor cells for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
Methods of promoting cardiac repair using growth factors fused to heparin binding sequences
ActiveUS20080138323A1More cartilageReduce the impactPeptide/protein ingredientsAntibody mimetics/scaffoldsDamages tissueBinding peptide
The present invention is directed to proteins in which a heparin binding peptide is fused to a growth factor that promotes cell growth and survival. The compound thus formed is bound to the surface of cells which are then administered to damaged tissue. The growth factor is thereby maintained at the site of administration where it promotes repair.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Biologic patch material for cardiac repair
The invention relates to a biologic patch material and concretely relates to a biologic patch material for cardiac repair. The biologic patch material comprises (i) at least two porous structural layers that are compounded together, wherein the porous structural layer comprises a first structural layer for preventing repairing cell loss and a second structural layer for loading (or accommodating) cardiac repairing cells and the repairing cells are selected from cardiac stem cells, cardiac progenitor (precursor) cells or their composition (derived from embryonic stem cells, induced pluripotent stem cells, heart tissue stem cells, bone marrow and peripheral blood), and (ii) repairing cells loaded on the second structural layer, wherein at least a part of or all the repairing cells are located in gaps of the second structural layer. The biologic patch material can load growing of repairing cells and has very good biocompatibility and mechanical strength.
Owner:SHANGHAI EAST HOSPITAL
Cardiac Repair By Reprogramming of Cardiac Fibroblasts Into Cardiomyocytes
InactiveUS20120213738A1Preventing delaying developmentAvoid delayBiocideOrganic active ingredientsGATA4Reprogramming
The present invention involves the use of transcription factors including Tbx5, Mef2C, Hand2, myocardin and Gata4 to reprogram cardiac fibroblasts into cardiomyocytes, both in vitro and in vivo. Such methods find particular use in the treatment of patients post-myocardial infarction to prevent or limit scarring and to promote myocardial repair.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods for isolating human cardiac ventricular progenitor cells
ActiveUS20180148691A1Potential variationSkeletal/connective tissue cellsUnknown materialsProgenitorPositive selection
The present invention provides methods for isolating human cardiac ventricular progenitor cells (HVPs), wherein cultures of day 5-7 cardiac progenitor cells are negatively selected for one or more first markers expressed on human pluripotent stem cells, such as TRA-1-60, to thereby isolate HVPs. The methods can further include positive selection for expression of a second marker selected from the group consisting of JAG1, FZD4, LIFR, FGFR3 and TNFSF9. Large populations, including clonal populations, of isolated HVPs that are first marker negative / second marker positive are also provided. Methods of in vivo use of the HVPs for cardiac repair or to improve cardiac function are also provided. Methods of using the HVPs for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
Cardiac repair by reprogramming of cardiac fibroblasts into cardiomyocytes
InactiveUS9017661B2Preventing delaying developmentAvoid delayOrganic active ingredientsBiocideGATA4Reprogramming
The present invention involves the use of transcription factors including Tbx5, Mef2C, Hand2, myocardin and Gata4 to reprogram cardiac fibroblasts into cardiomyocytes, both in vitro and in vivo. Such methods find particular use in the treatment of patients post-myocardial infarction to prevent or limit scarring and to promote myocardial repair.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cardioprotective effects of GHRH agonists
ActiveUS8507433B1Improved cardiac structureFunction increasePeptide/protein ingredientsAntinoxious agentsInsulin-like growth factorFunctional decline
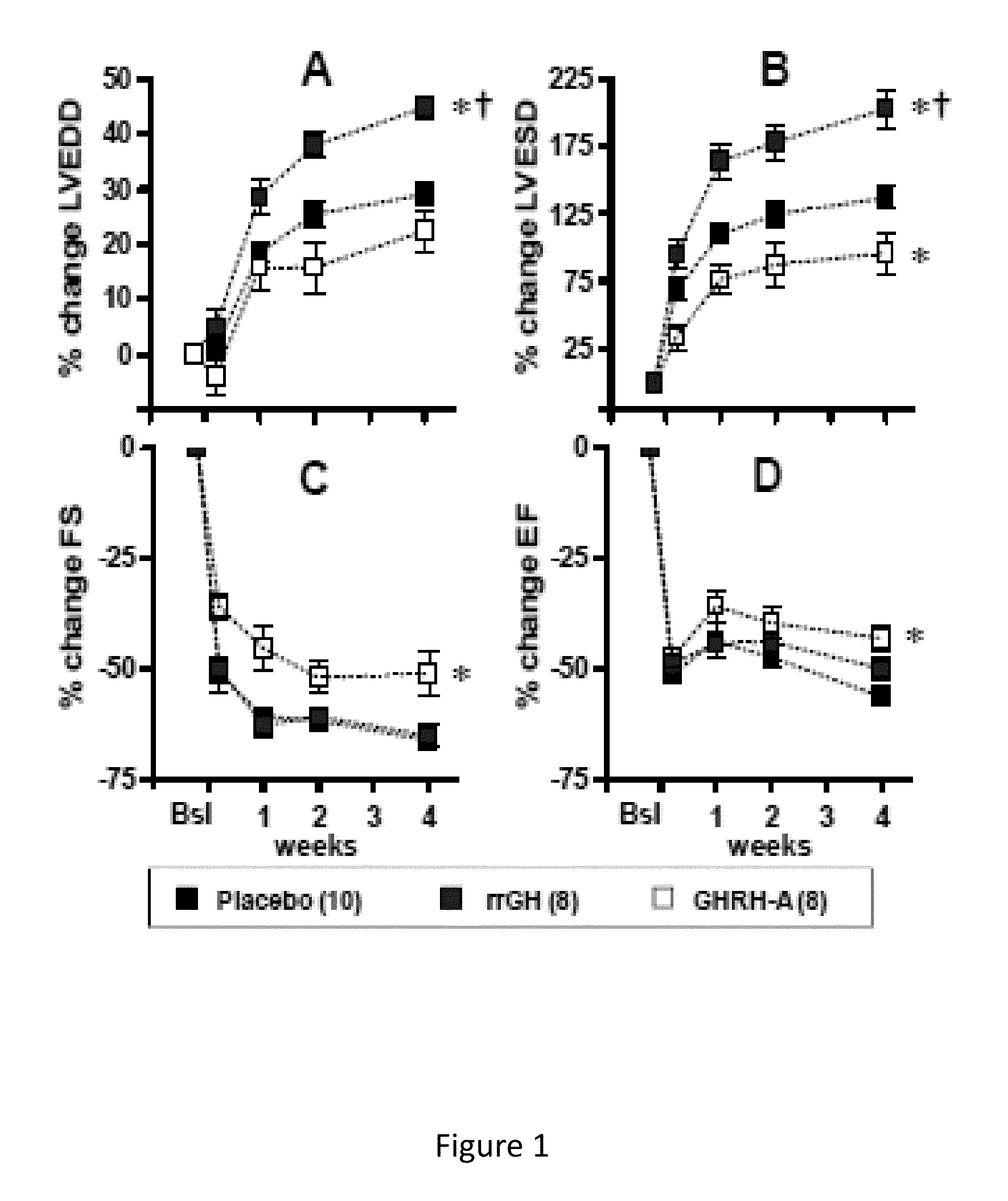
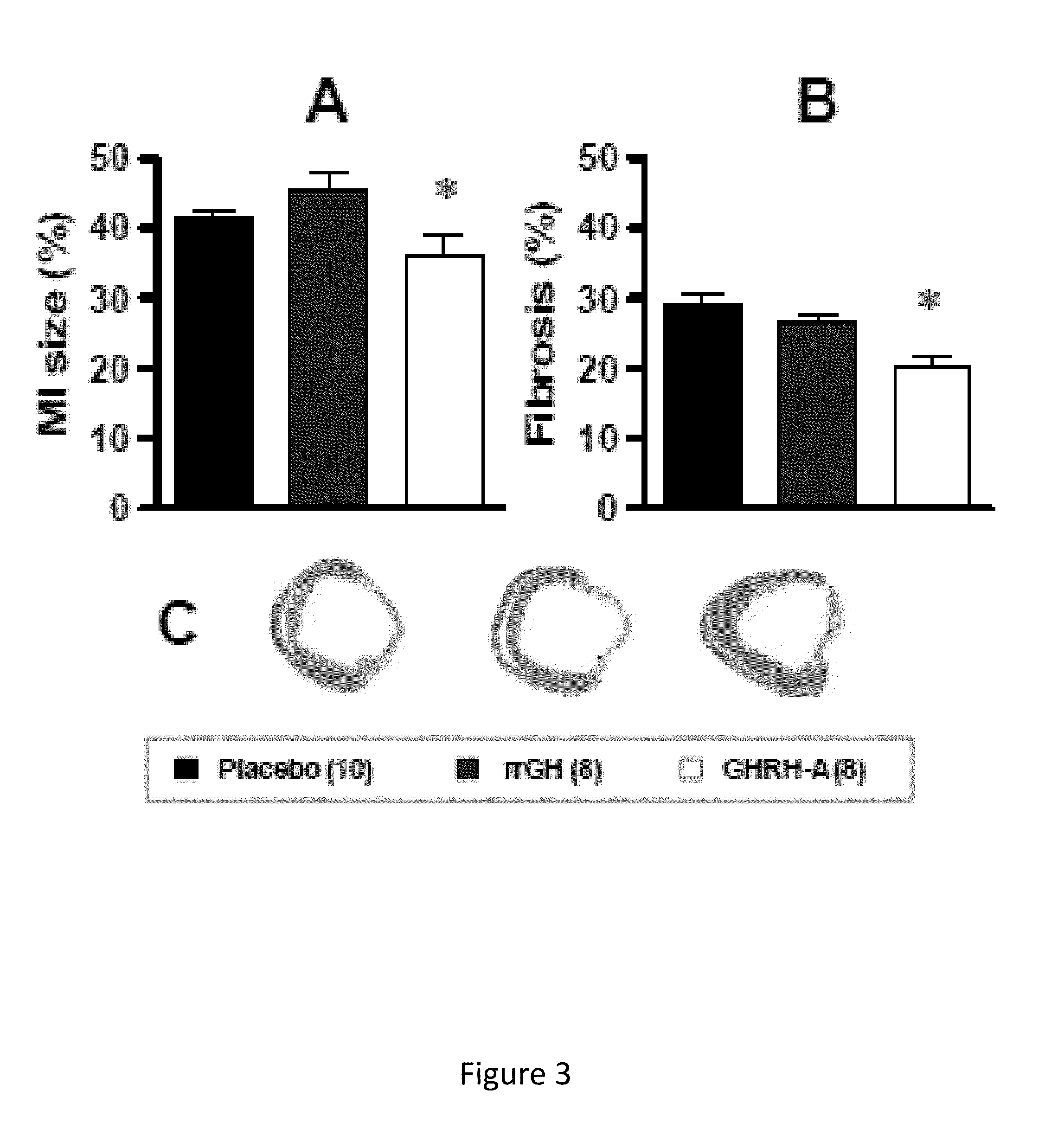
Whether the growth hormone (GH) / Insulin-like growth factor 1(IGF-I) axis exerts cardioprotective effects remains controversial; and the underlying mechanism(s) for such actions are unclear. Here we tested the hypothesis that growth-hormone releasing hormone (GHRH) directly activates cellular reparative mechanisms within the injured heart, in a GH / IGF-I independent fashion. Following experimental myocardial infarction (MI), rats were randomly assigned to receive, during a 4 week period, either placebo (n=14), rat recombinant GH (rrGH, n=8) or JI-38 (n=8; 50 μg / Kg / day), a potent GHRH-agonist. JI-38 did not elevate serum levels of GH or IGF-I, but markedly attenuated the degree of cardiac functional decline and remodeling after injury. In contrast, GH administration markedly elevated body weight, heart weight, circulating GH and IGF-I, but did not offset the decline in cardiac structure and function. Whereas, both JI-38 and GH augmented levels of cardiac precursor cell proliferation, only JI-38 increased anti-apoptotic gene expression. The receptor for GHRH was detectable on myocytes supporting direct activation of cardiac signal transduction. Collectively, these findings demonstrate that within the heart GHRH-agonists can activate cardiac repair following MI, suggesting the existence of a potential signaling pathway based on GHRH in the heart. The phenotypic profile of the response to a potent GHRH agonist has therapeutic implications.
Owner:MIAMI UNIVERISTY OF +1
Thermally-induced phase separation nanofiber tubular stent and preparation method thereof
InactiveCN106075594AImprove adhesionPromote proliferationPharmaceutical delivery mechanismTissue regenerationFiberBursting strength
The invention discloses an acellular dermis matrix enhanced thermally-induced phase separation nanofiber small-bore tubular stent and a preparation method thereof. The preparation method comprises the following steps: performing decellularization treatment on aorta of healthy animals; placing the matrix obtained by decellularization on a watch glass, dropwise adding ultrapure water to submerge all the matrix, and placing in a refrigerator for freezing; spinning nanofibers onto stainless steel shaft core of a mold, putting whole nanofiber and the shaft core into a vacuum drying oven for drying; sheathing the outer layer of the nanofiber with the tubular matrix as a mechanical reinforcing layer; and assembling the compounded double-layer stent together with the shaft core into the mold, and performing solution casting and treatment to obtain the tubular stent. The prepared tubular stent has good mechanical performance, bursting strength and biocompatibility, has no hypersensitivity on a human body in transplanting operation, and has affinity with the blood and peripheral tissues in a human body, thus having good application prospects in cardiovascular and cardiac repair surgeries.
Owner:DONGHUA UNIV
Use of human stem cells and/or factors they produce to promote adult mammalian cardiac repair through cardiomyocyte cell division
InactiveUS20070072294A1RestoreLarge workGenetically modified cellsCell culture supports/coatingDiseaseMammal
A method for treating a subject afflicted with a cardiac disorder, in vivo, comprising (i) producing a solution comprising media conditioned from the culture of cells, in vitro, and (ii) administering the solution of step (i) to the subject, thereby treating the cardiac disorder in the subject. Methods for determining whether an agent stimulates or inhibits myocyte proliferation.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
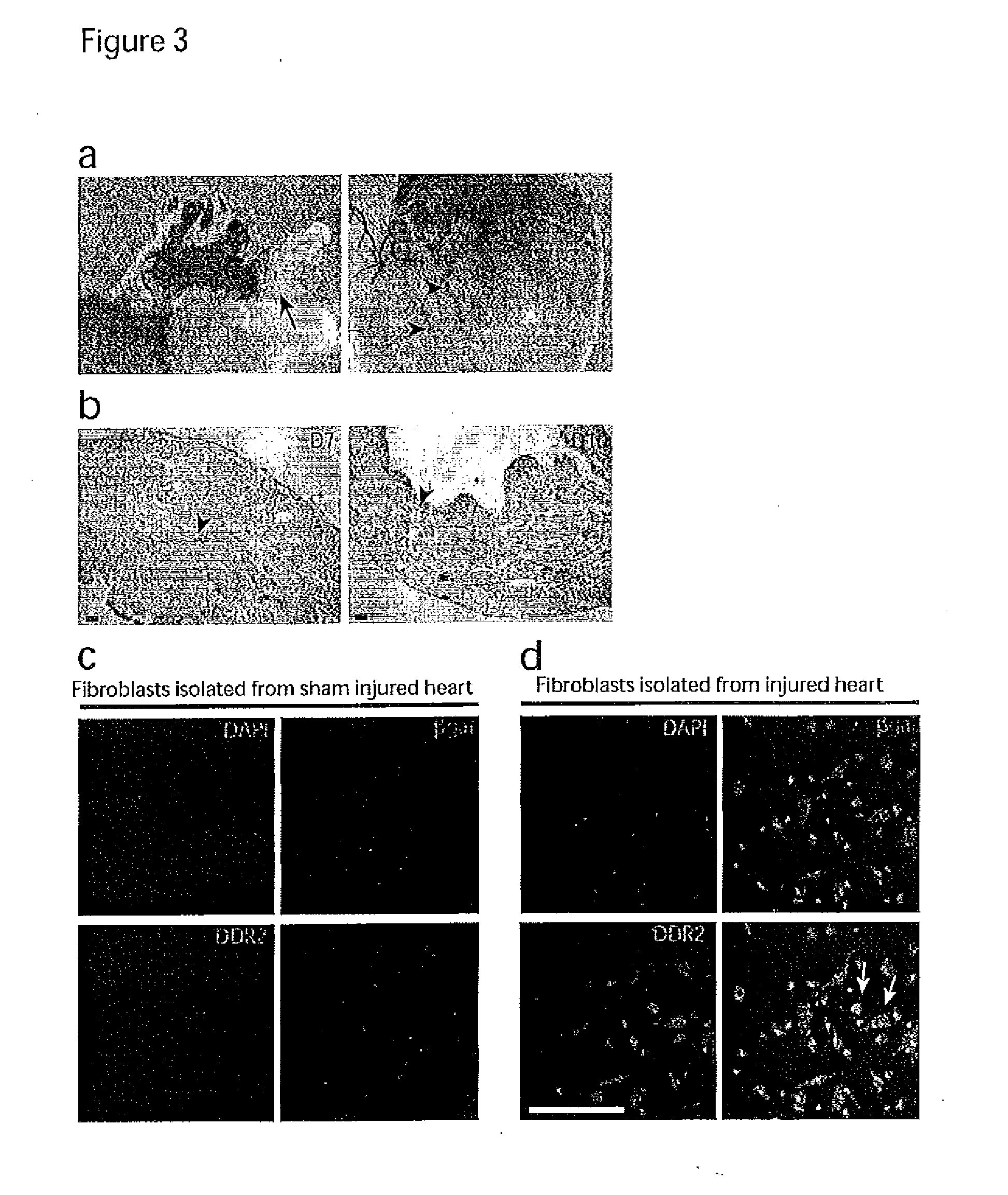
Method for sorting cardiac fibroblast CD90<+> subsets of mice and application of cardiac fibroblast CD90<+> subsets
The invention belongs to the animal cell culture techniques and relates to a method for sorting cardiac fibroblast CD90<+> subsets of mice and application of the cardiac fibroblast CD90<+> subsets. The method comprises the following steps: carrying out recovery and enlarged culture on mice cardiac fibroblast cell strains; sorting cardiac fibroblast CD90<+> subsets by virtue of a PE-marked CD90 antibody; collecting the sorted cardiac fibroblast CD90<+> subsets, carrying out centrifugation to remove supernatant so as to obtain precipitates, inoculating the precipitates into a culture bottle, carrying out enlarged culture, so as to finish the sorting of the cardiac fibroblast CD90<+> subsets of the mice. The sorted cardiac fibroblast CD90<+> subsets are taken as preponderant subsets for sorting cardiac fibroblasts and are used for triggering the myocardium repair potential of the cardiac fibroblasts, so that the experimental basis and theoretical support are provided for the efficient utilization of the cardiac fibroblasts.
Owner:XINXIANG MEDICAL UNIV
Compositions and methods for cardiac repair
PendingUS20190175690A1Reduce cell deathReducing cardiomyocyte loss and cell deathOrganic active ingredientsPeptide/protein ingredientsCell biologySmall molecule
The present invention features compositions comprising a modified RNA encoding a Yes-associated protein (YAP) polypeptide and methods of using such compositions for cardiac repair. In particular embodiments, a modified RNA encoding a YAP polypeptide is administered in combination with an agent that reduces YAP degradation, such as a small molecule E64d.
Owner:CHILDRENS MEDICAL CENT CORP
Pharmacological Delivery Implement for Use with Cardiac Repair Devices
A pharmacological delivery implement for use with cardiac repair devices. The pharmacological delivery implement comprises a porous member defining an outer surface, an internal channel configured to selectively house a pharmacological agent, and a plurality of release holes each extending from the outer surface to the internal channel. Following implantation of the pharmacological delivery implement, at least a portion of the pharmacological agent exits the internal channel through the plurality of release holes.
Owner:MEDTRONIC INC
Cardioprotective effects of ghrh agonists
InactiveUS20140057847A1Function increaseSimple structurePeptide/protein ingredientsDepsipeptidesAnti apoptotic genesFunctional decline
Disclosed herein are methods demonstrating that growth-hormone releasing hormone (GHRH) directly activates cellular reparative mechanisms within the injured heart, in a GH / IGF-I independent fashion. Following experimental myocardial infarction (MI), rats were randomly assigned to receive, during a 4 week period, either placebo (n=14), rat recombinant GH (rrGH, n=8) or JI-38 (n=8; 50 μg / Kg / day), a potent GHRH-agonist. JI-38 did not elevate serum levels of GH or IGF-I, but markedly attenuated the degree of cardiac functional decline and remodeling after injury. In contrast, GH administration markedly elevated body weight, heart weight, circulating GH and IGF-I, but did not offset the decline in cardiac structure and function. Whereas, both JI-38 and GH augmented levels of cardiac precursor cell proliferation, only JI-38 increased anti-apoptotic gene expression. Collectively, these findings demonstrate that within the heart, GHRH-agonists can activate cardiac repair following MI.
Owner:UNIV OF MIAMI +1
Use of jagged 1/frizzled 4 as a cell surface marker for isolating human cardiac ventricular progenitor cells
ActiveUS10597637B2Easy and rapid isolationFunction increaseDrug screeningSkeletal/connective tissue cellsSurface markerCardiac functioning
The present invention provides Jagged 1 and Frizzled 4 as cell surface markers for isolating human cardiomyogenic ventricular progenitor cells, in particular progenitor cells that preferentially differentiate into cardiac ventricular muscle cells. Thus, the invention provides human ventricular progenitor (HVP) cells. The invention provides in vitro methods of the separation of Islet 1+ Jagged 1+ ventricular progenitor cells and / or Islet 1+ / Frizzled 4+ ventricular progenitor cells and / or Islet 1+ / Jagged 1+ / Frizzled 4+ ventricular progenitor cells, and the large scale expansion and propagation thereof. Large clonal populations of isolated Jagged 1+ and / or Frizzled 4+ventricular progenitor cells are also provided. Methods of in vivo use of Jagged 1+ and / or Frizzled 4+ ventricular progenitor cells for cardiac repair or to improve cardiac function are also provided. Methods of using the Jagged 1+ and / or Frizzled 4+ ventricular progenitor cells for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
Methods for isolating human cardiac ventricular progenitor cells
The present invention provides methods for isolating human cardiac ventricular progenitor cells (HVPs), wherein cultures of day 5-7 cardiac progenitor cells are negatively selected for one or more first markers expressed on human pluripotent stem cells, such as TRA-1-60, to thereby isolate HVPs. The methods can further include positive selection for expression of a second marker selected from the group consisting of JAG1, FZD4, LIFR, FGFR3 and TNFSF9. Large populations, including clonal populations, of isolated HVPs that are first marker negative / second marker positive are also provided. Methods of in vivo use of the HVPs for cardiac repair or to improve cardiac function are also provided. Methods of using the HVPs for cardiac toxicity screening of test compounds are also provided.
Owner:PROCELLA THERAPEUTICS AB
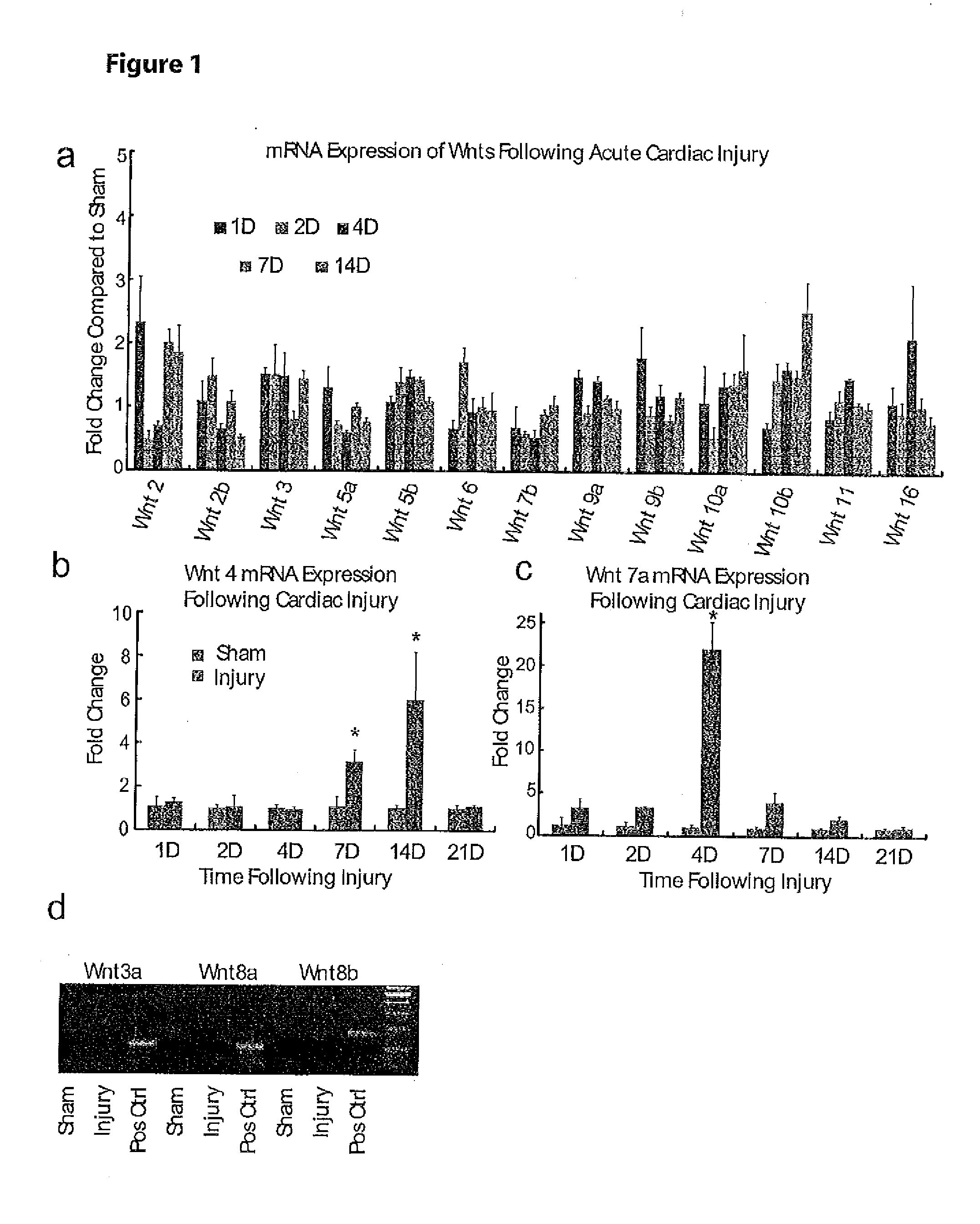
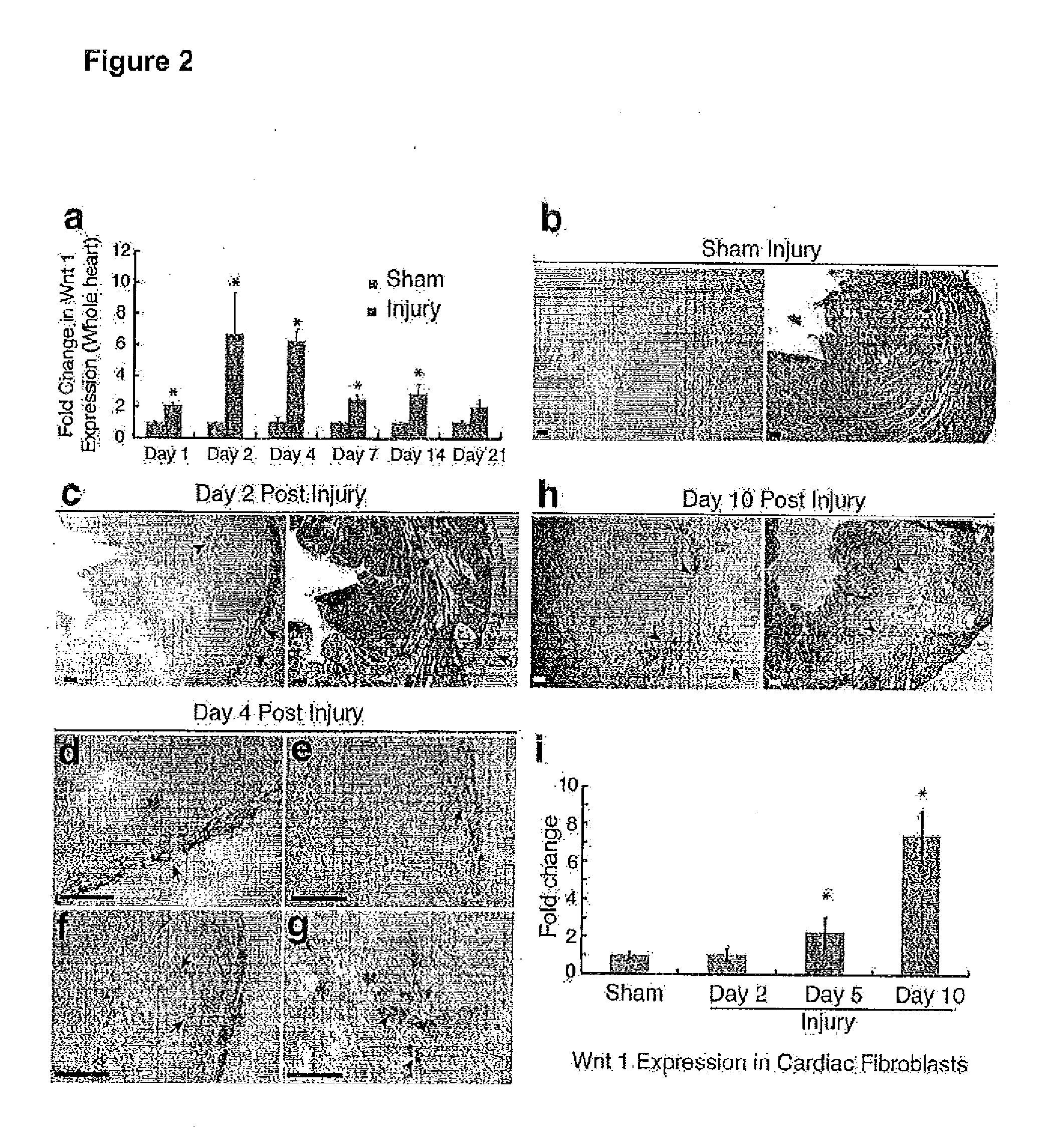
Wnt1 for Treatment of Cardiovascular Disorders and Injuries
ActiveUS20130309211A1Increased blood vessel formationIncrease blood flowBiocideOrganic active ingredientsProgenitorWNT1
The present invention relates to the discovery of the role of Wnt1 in multiple cardiovascular processes, including cardiac repair, angiogenesis, and stimulation of endothelial progenitor cells. This discovery provides methods of using Wnt1 to treat cardiovascular disorders and injuries.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com