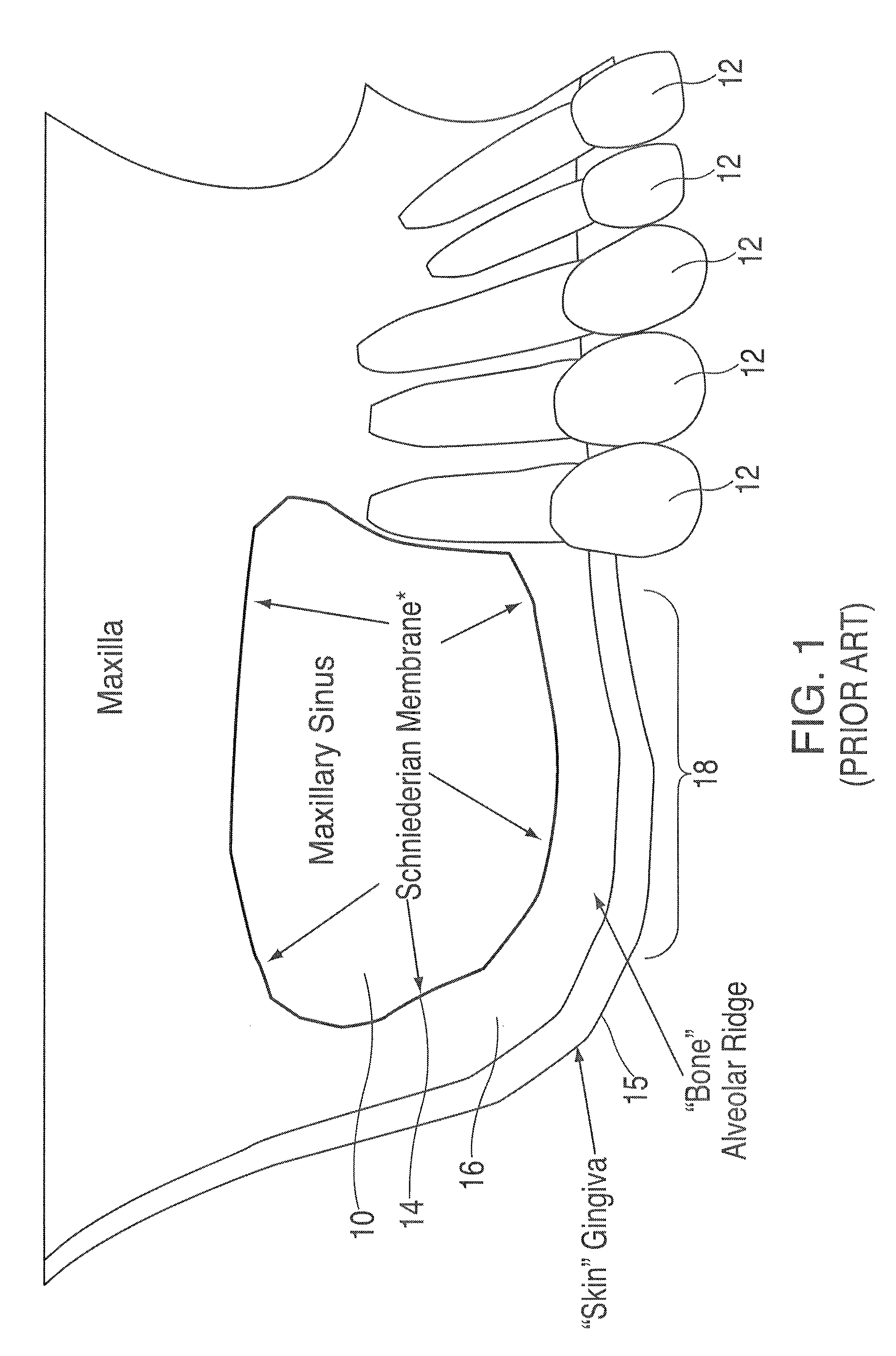
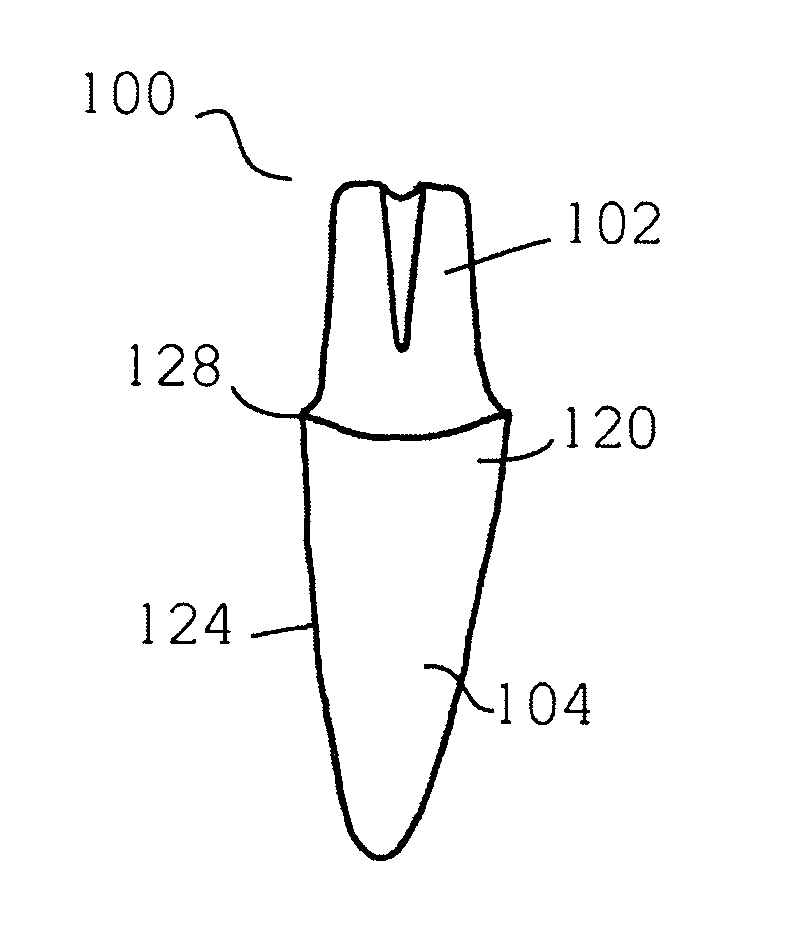
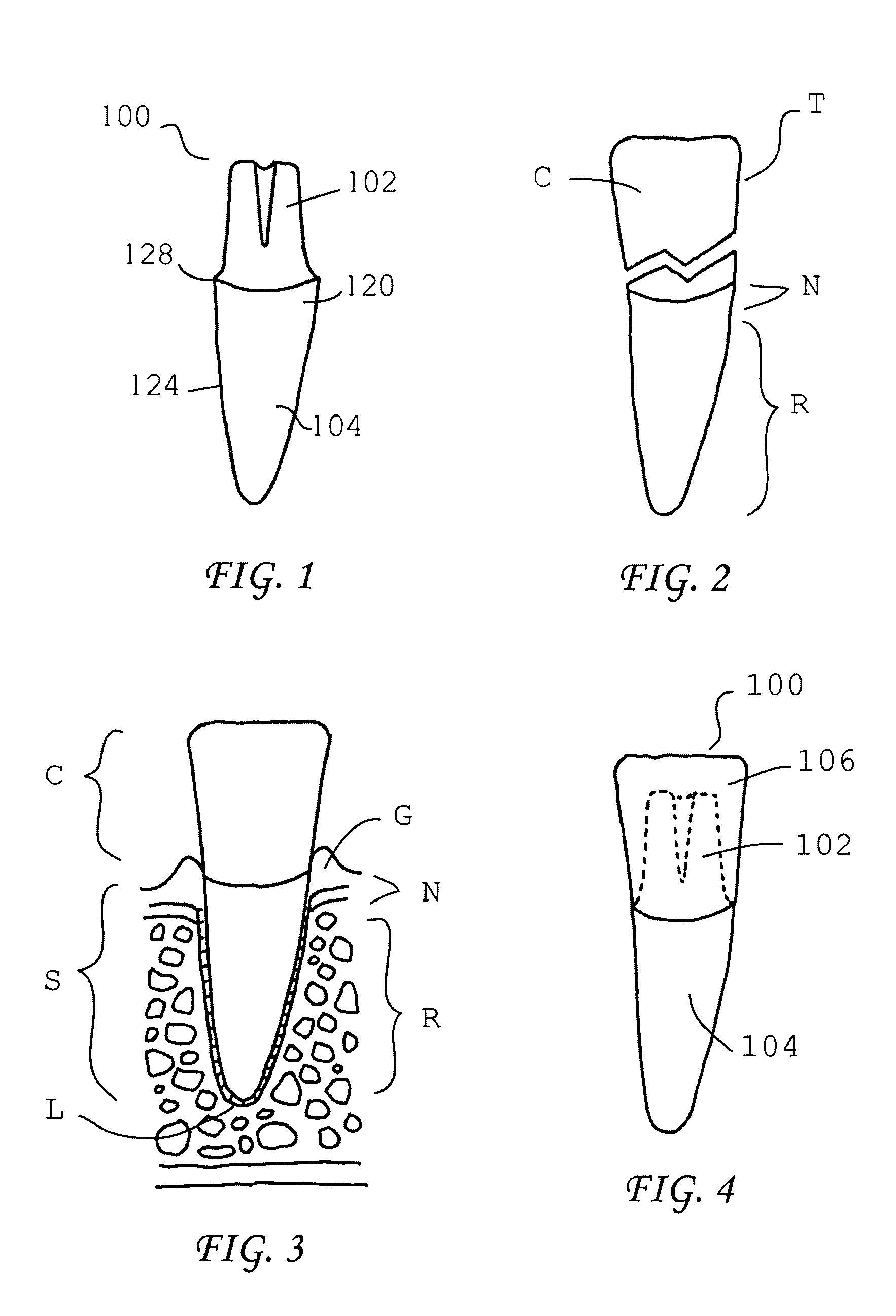
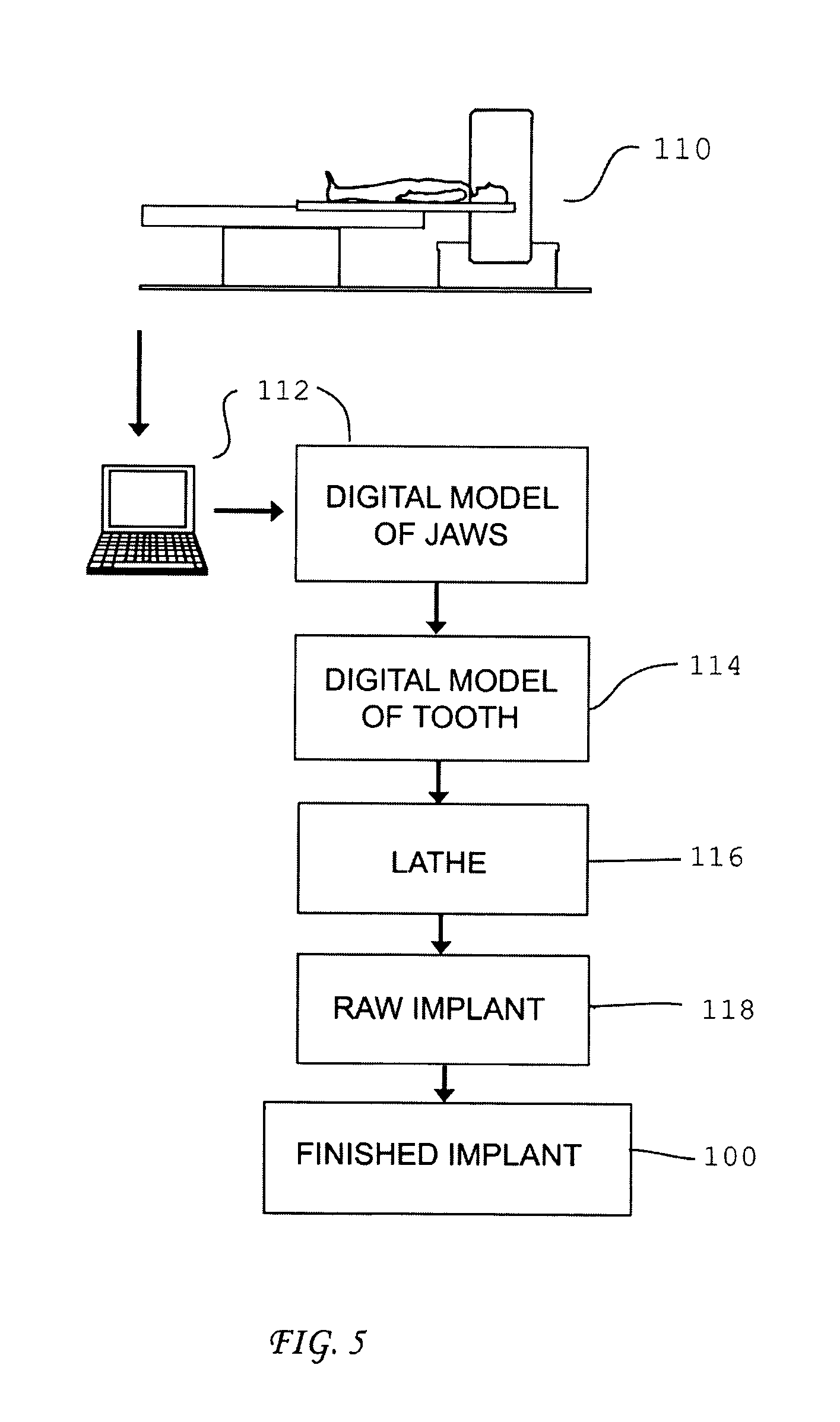
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
162 results about "Bones growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Osteoblasts, osteocytes and osteoclasts are the three cell types involved in the development, growth and remodeling of bones. Osteoblasts are bone-forming cells, osteocytes are mature bone cells and osteoclasts break down and reabsorb bone.
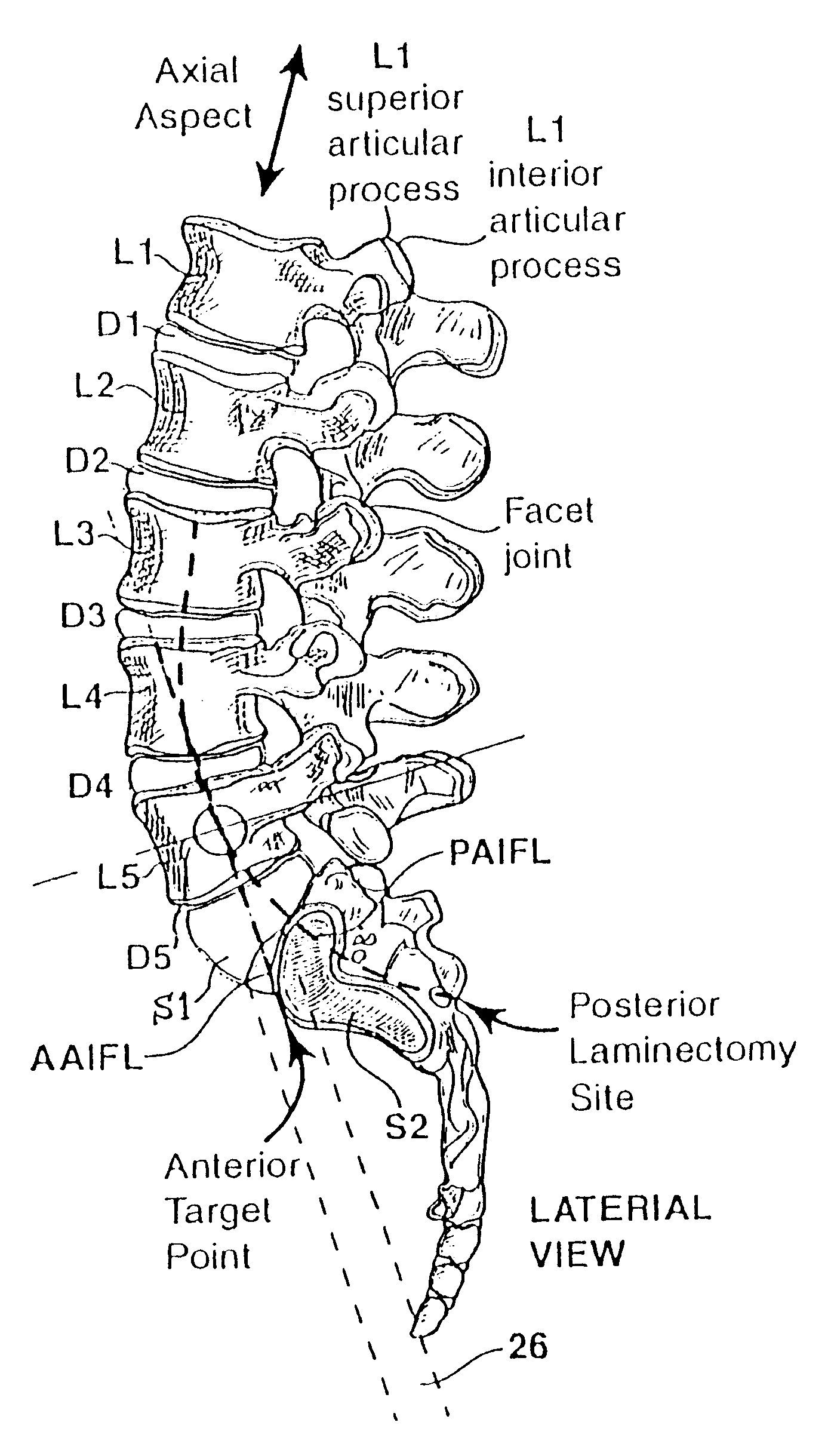
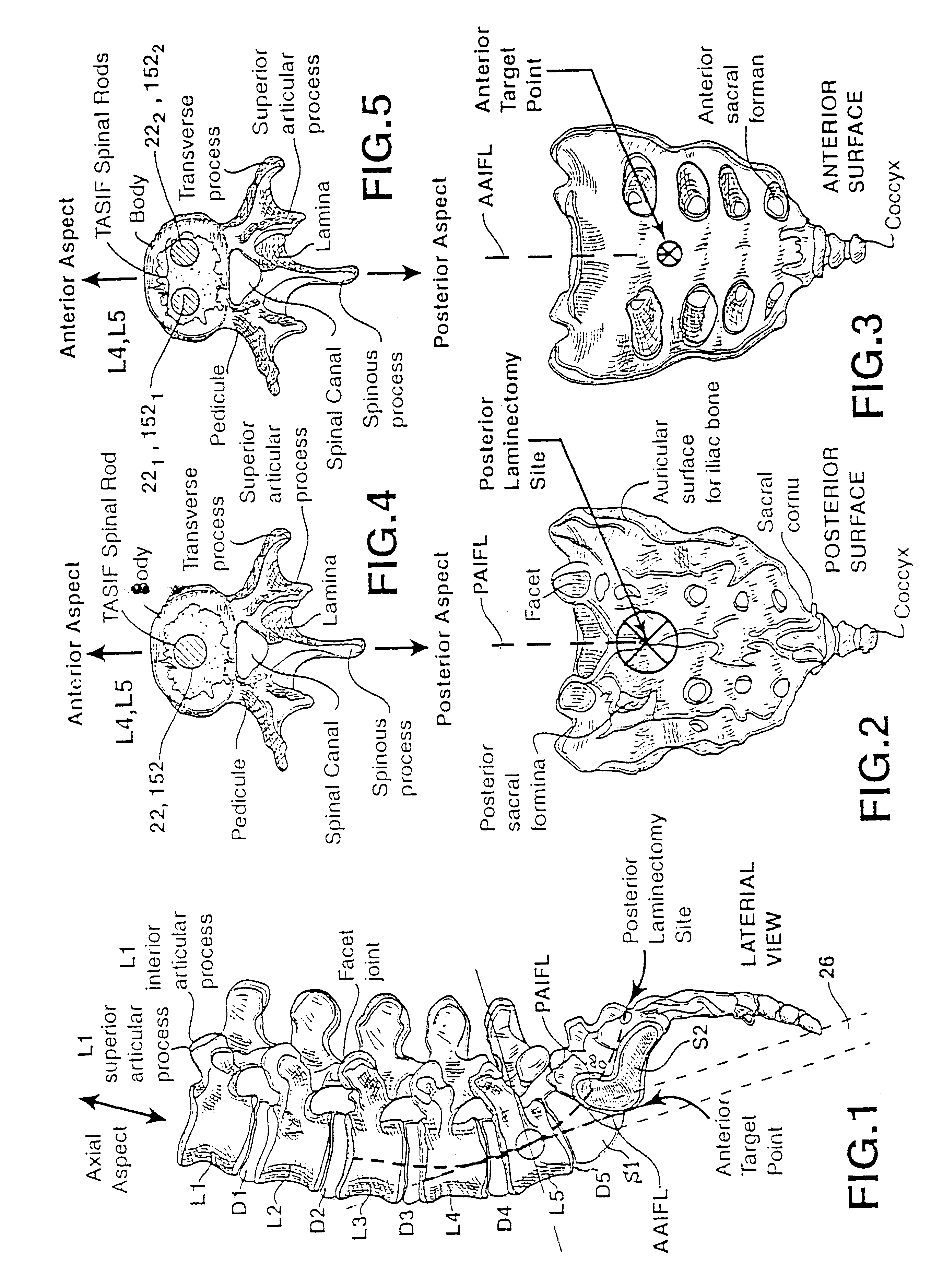
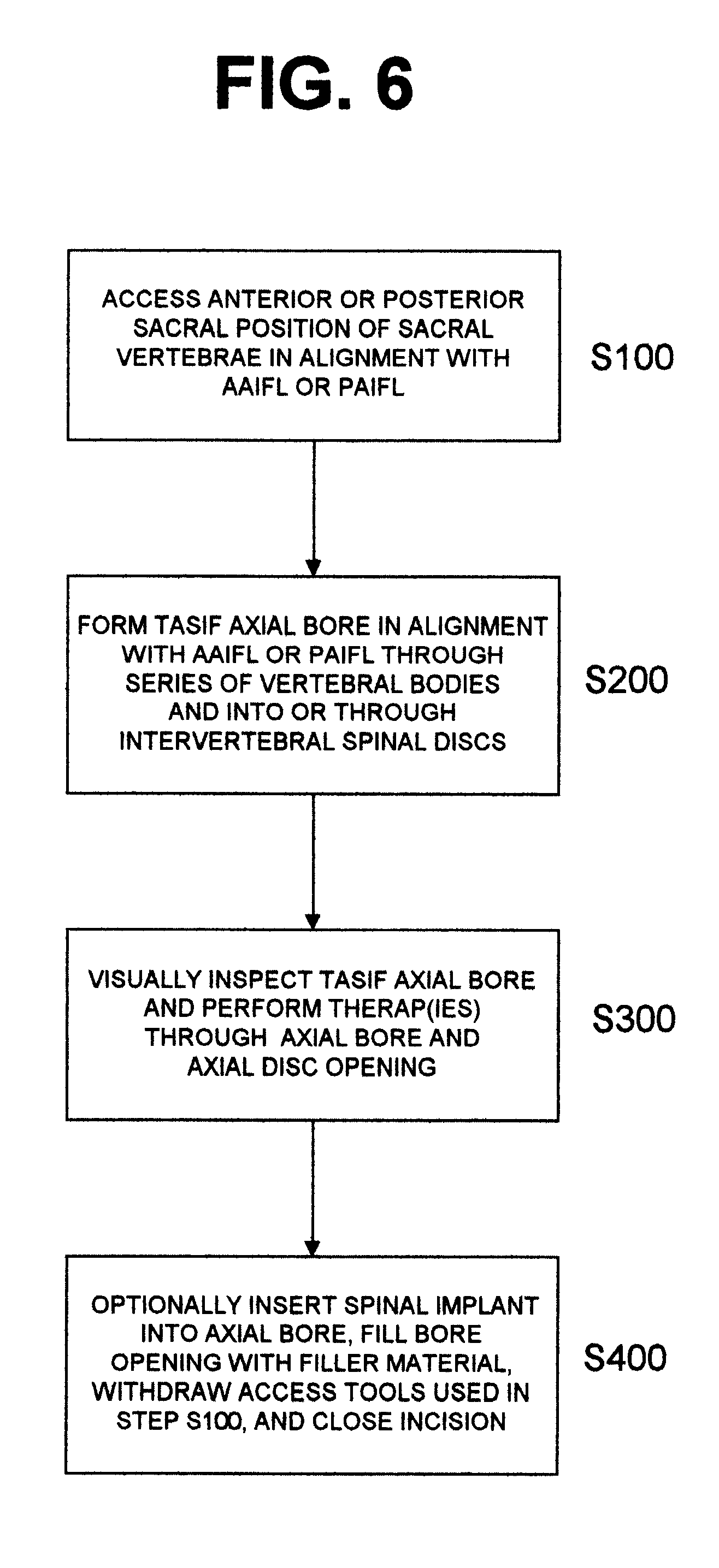
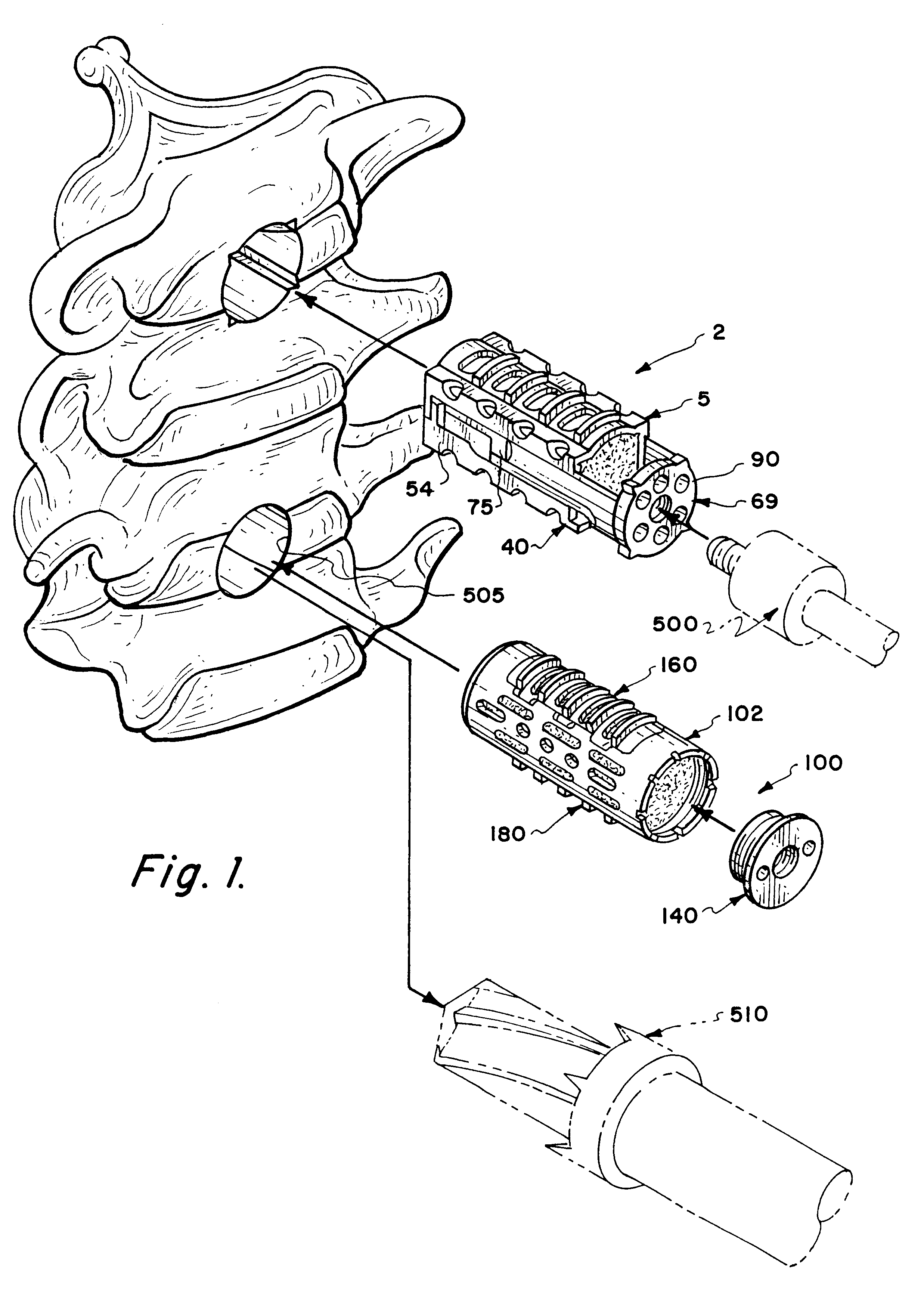
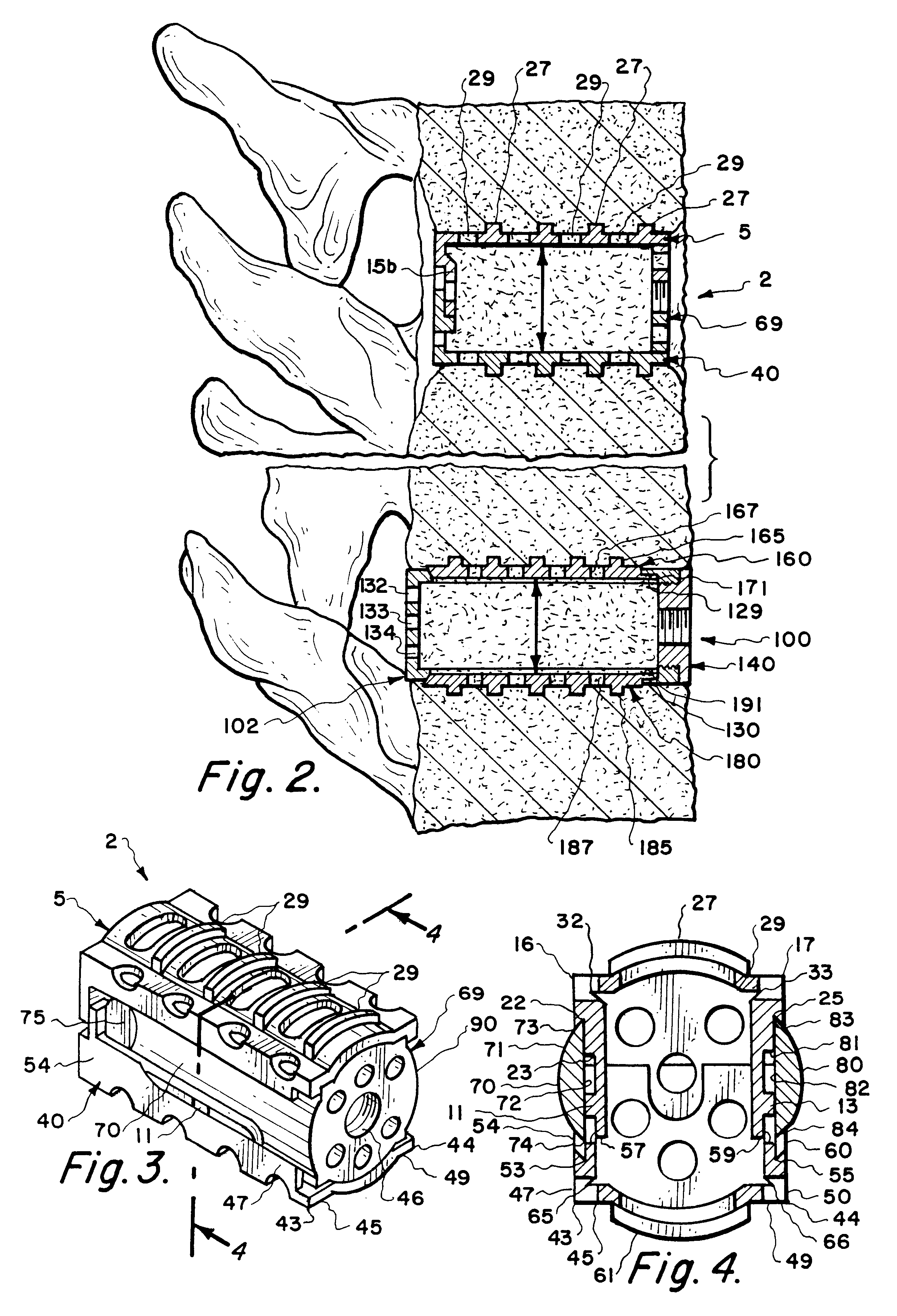
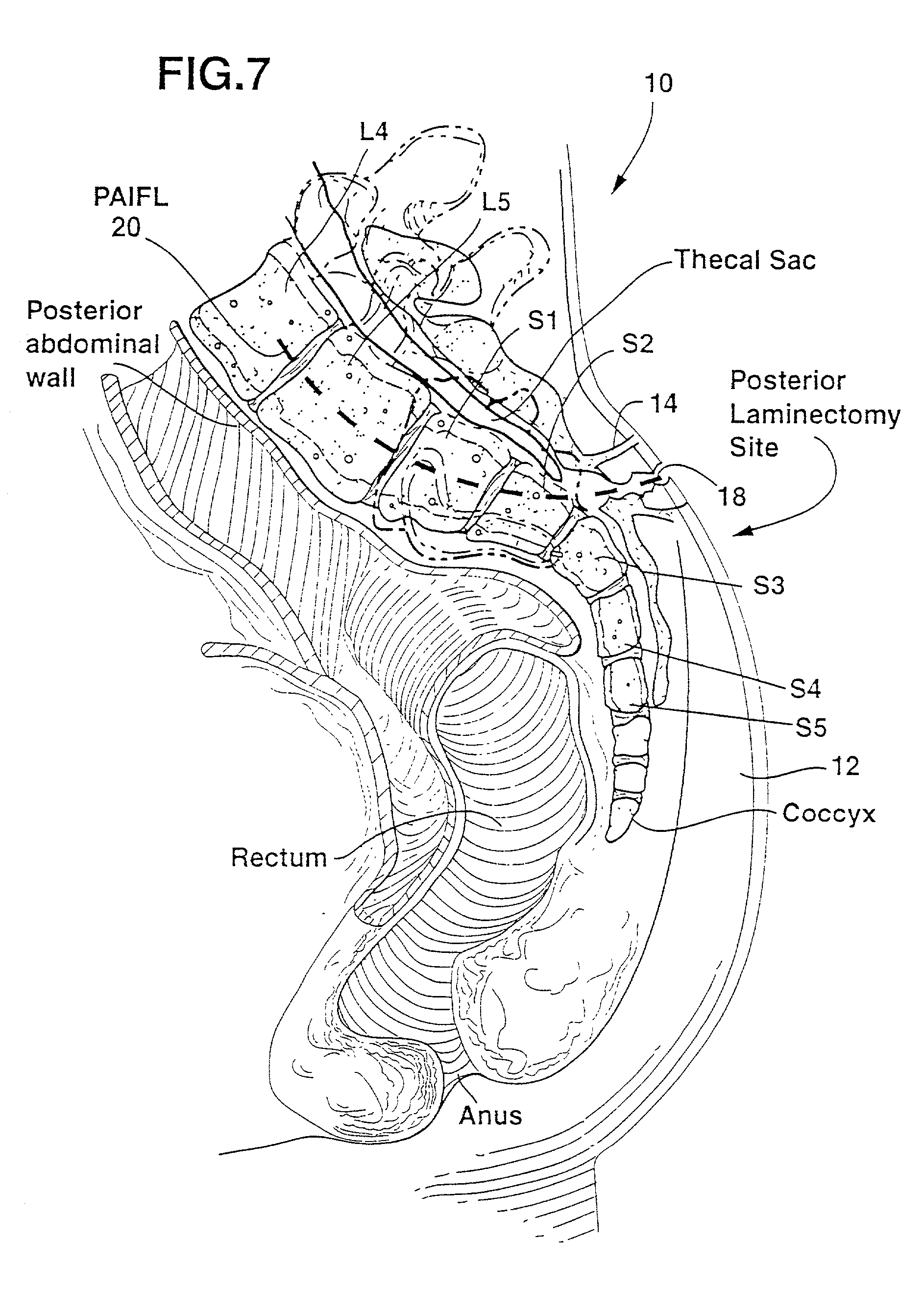
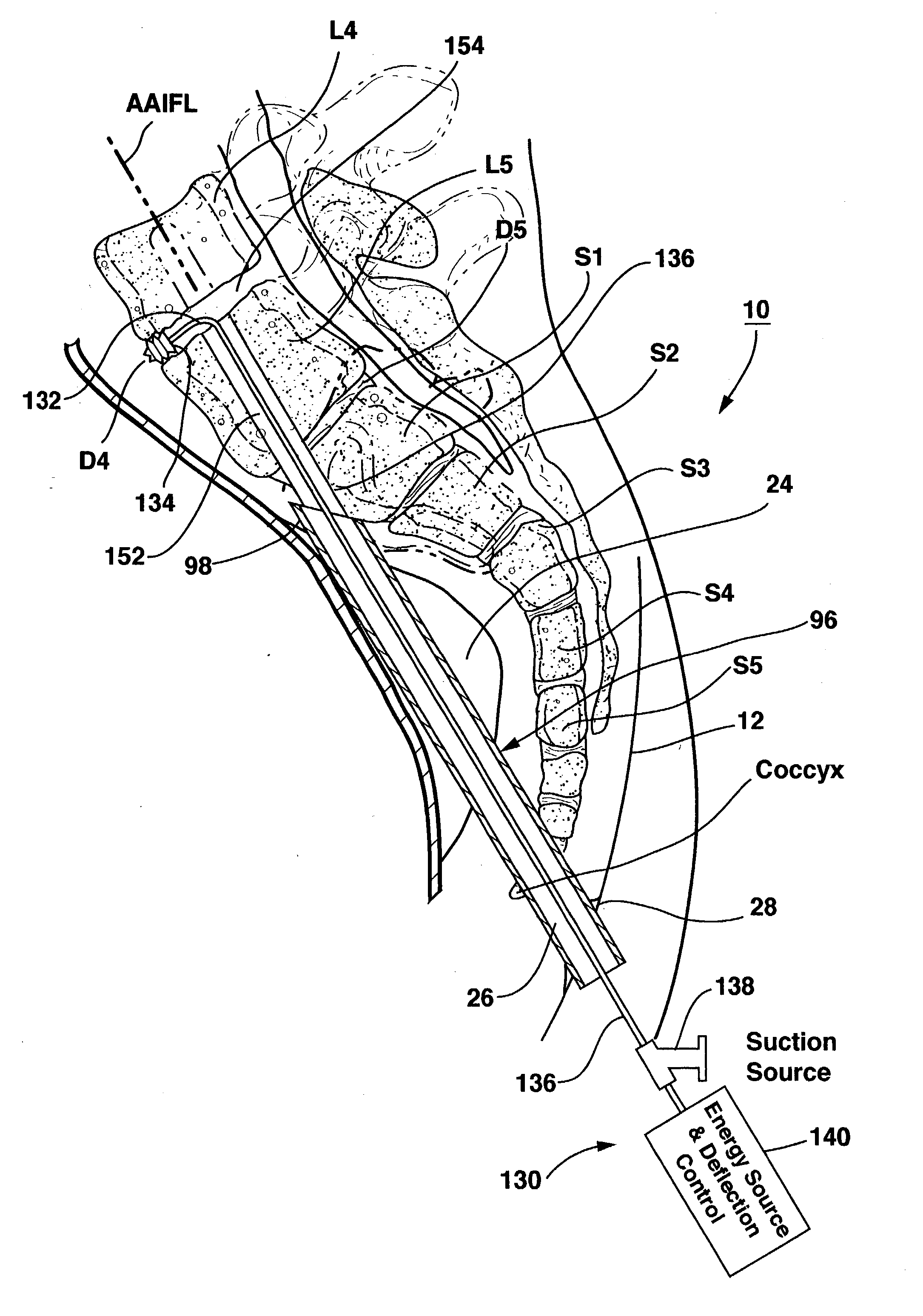
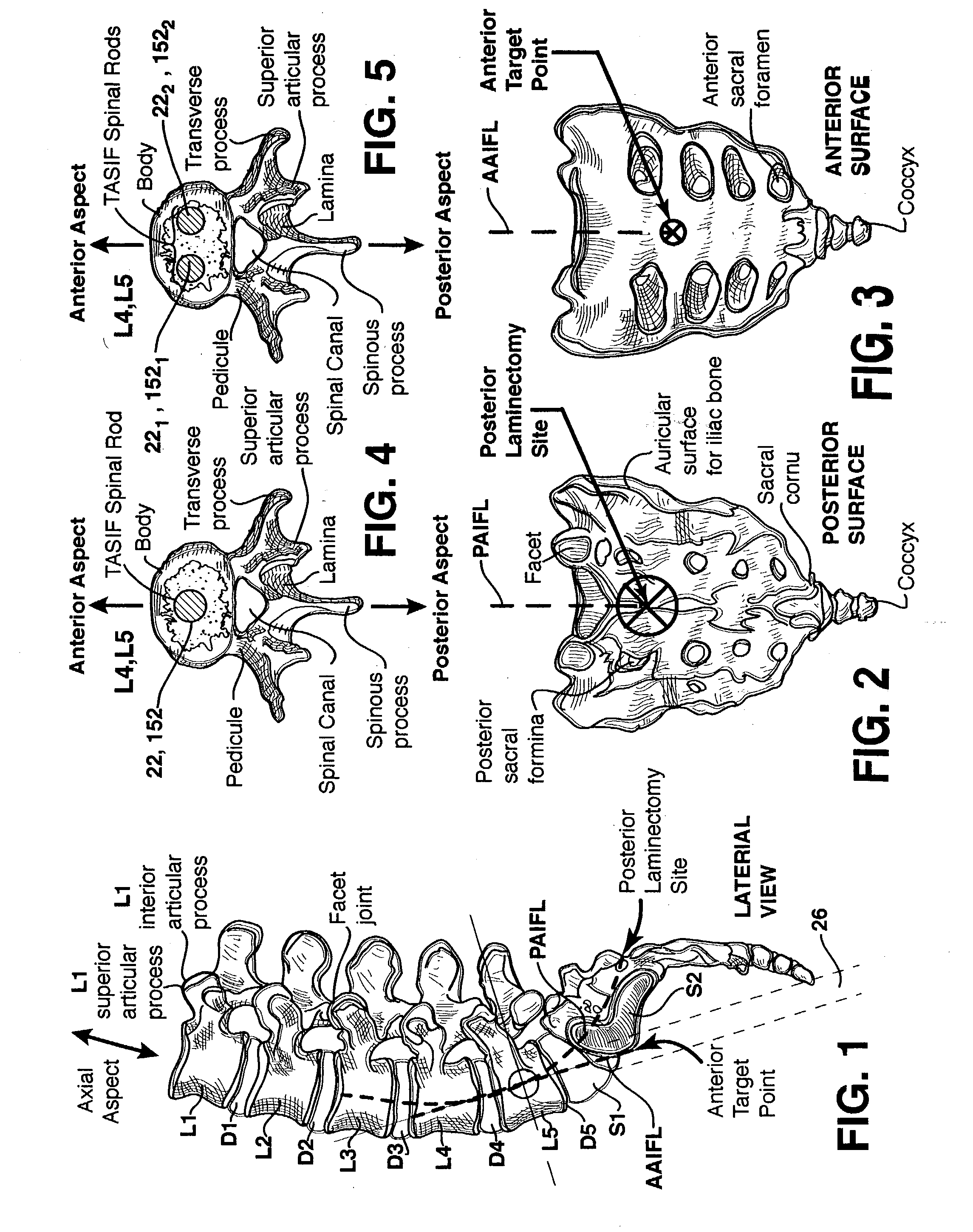
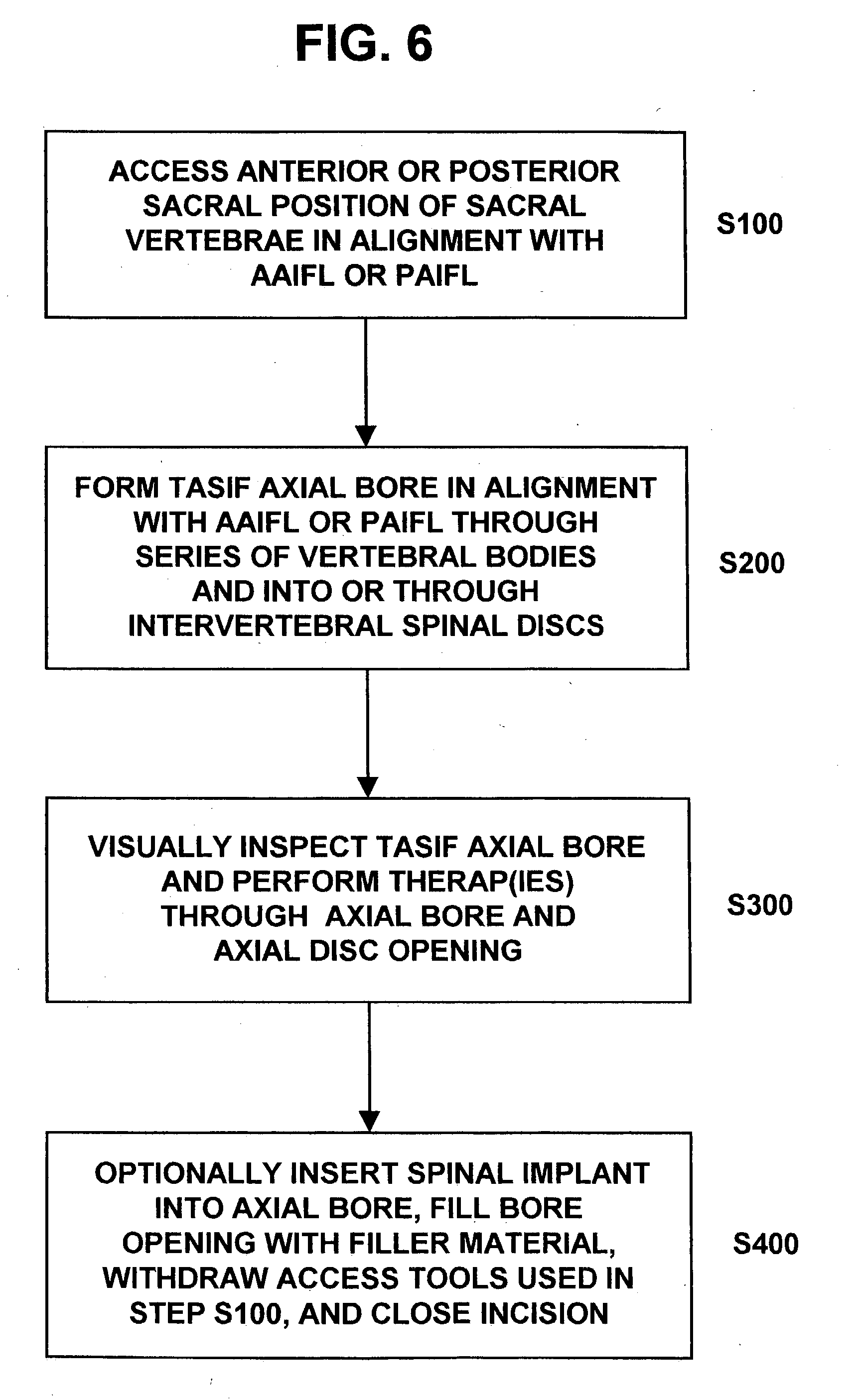
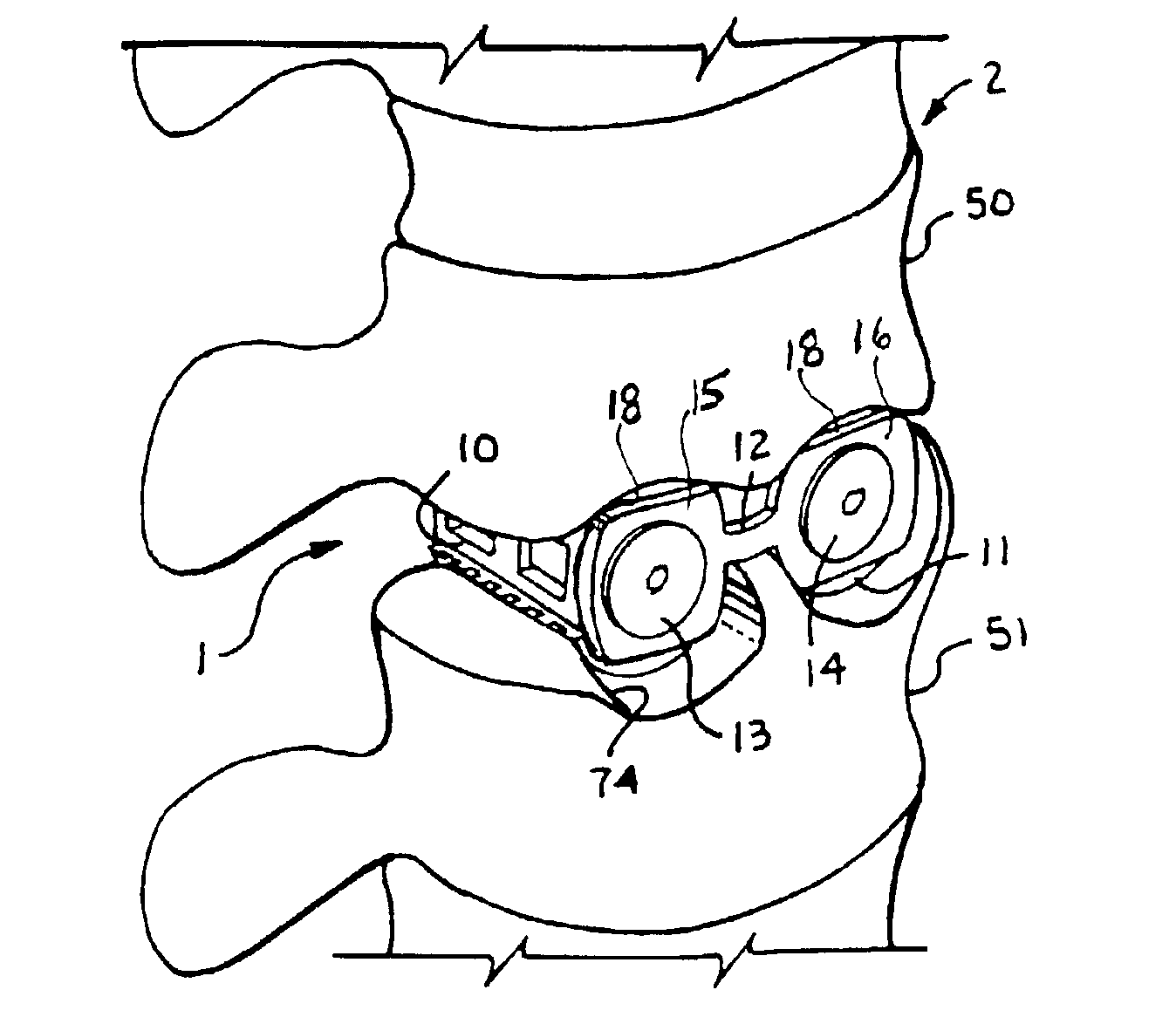
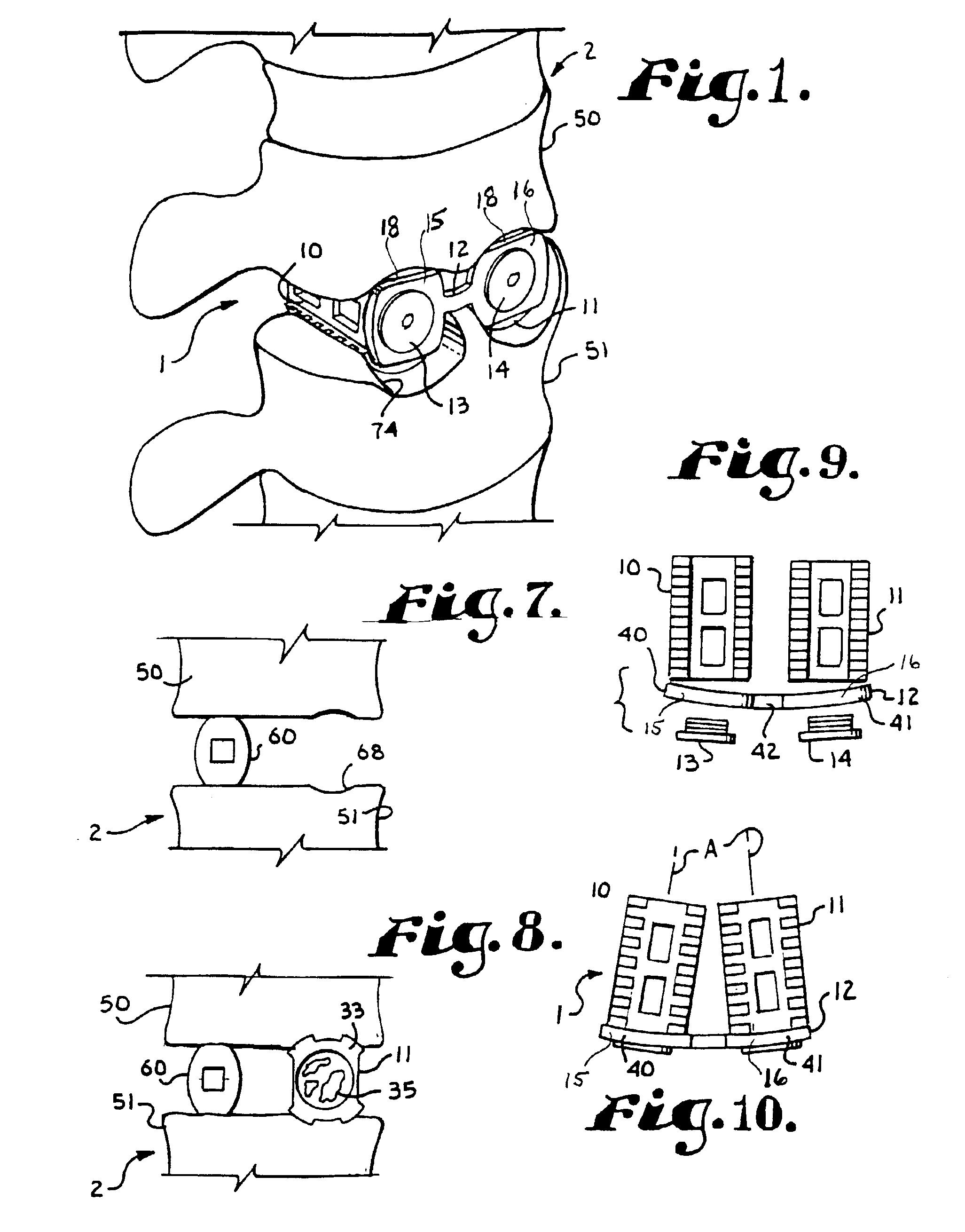
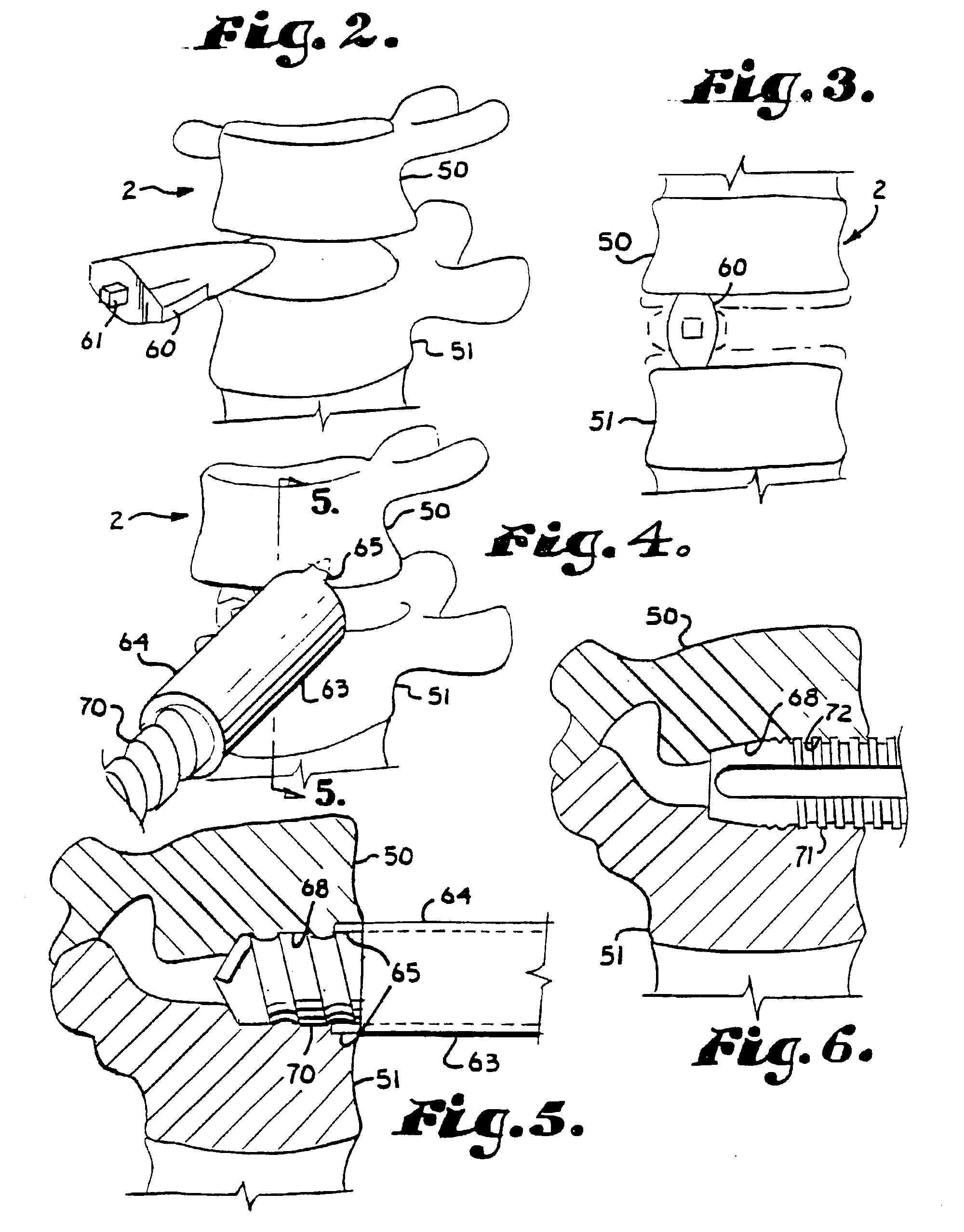
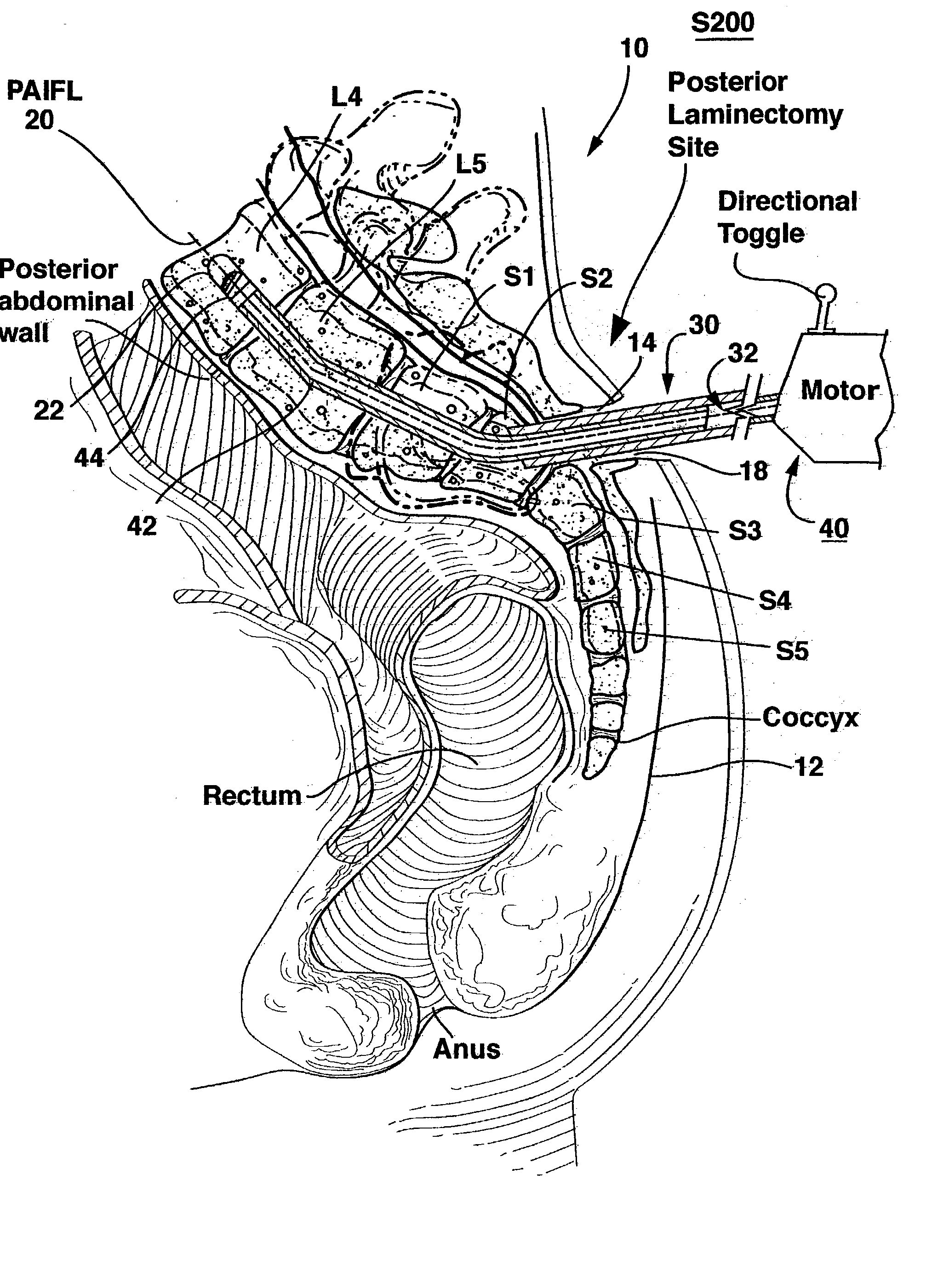
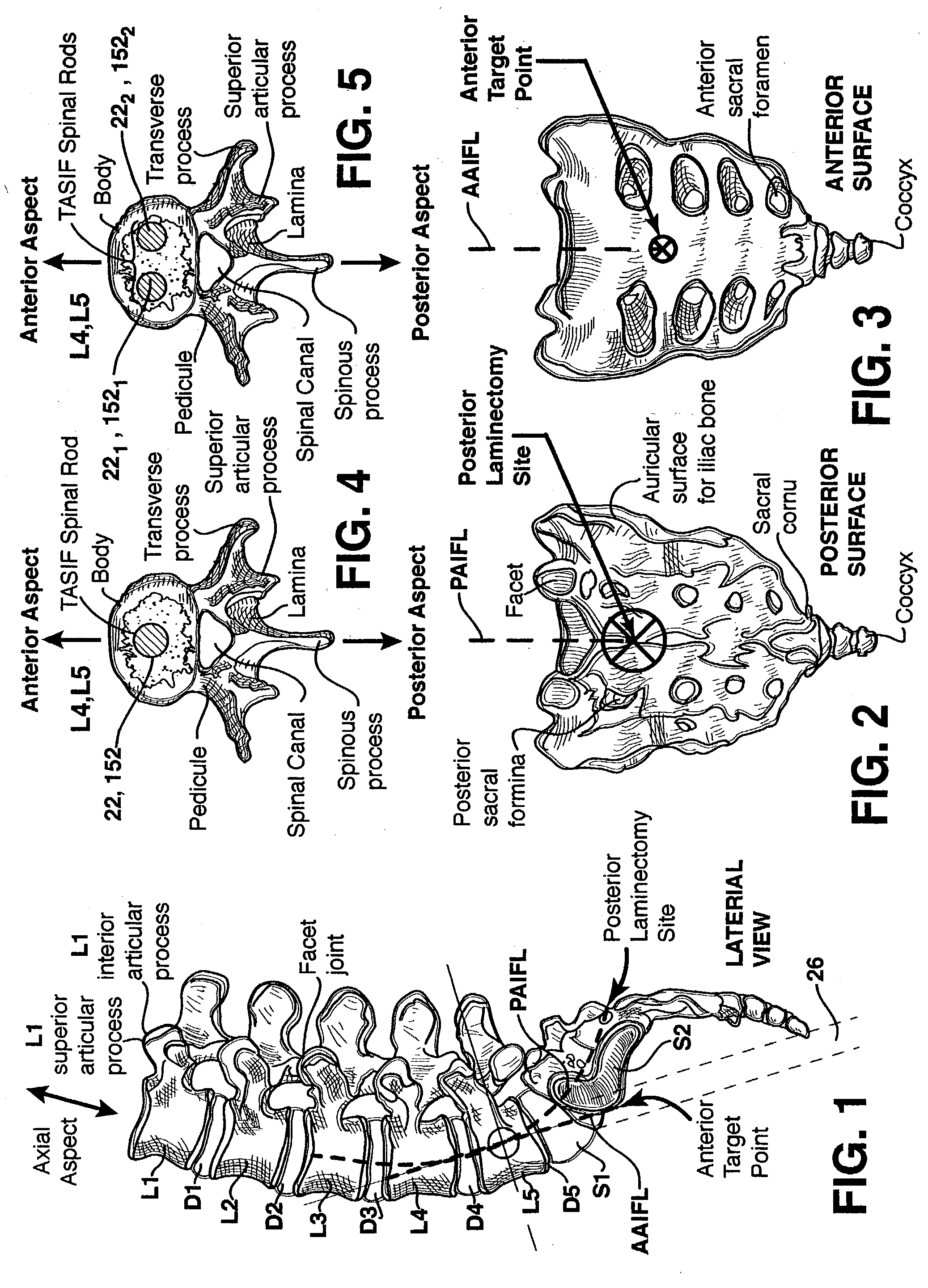
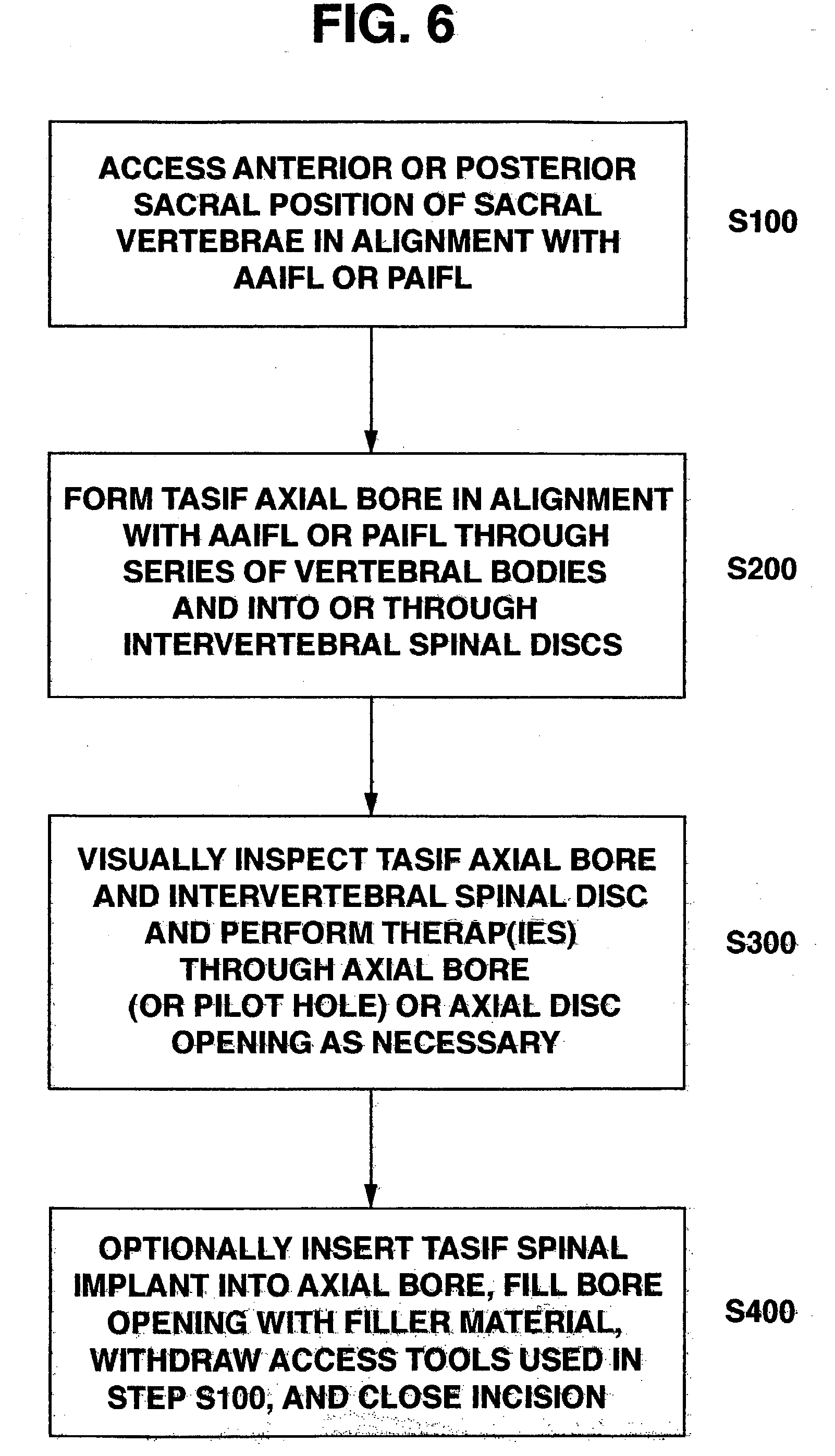
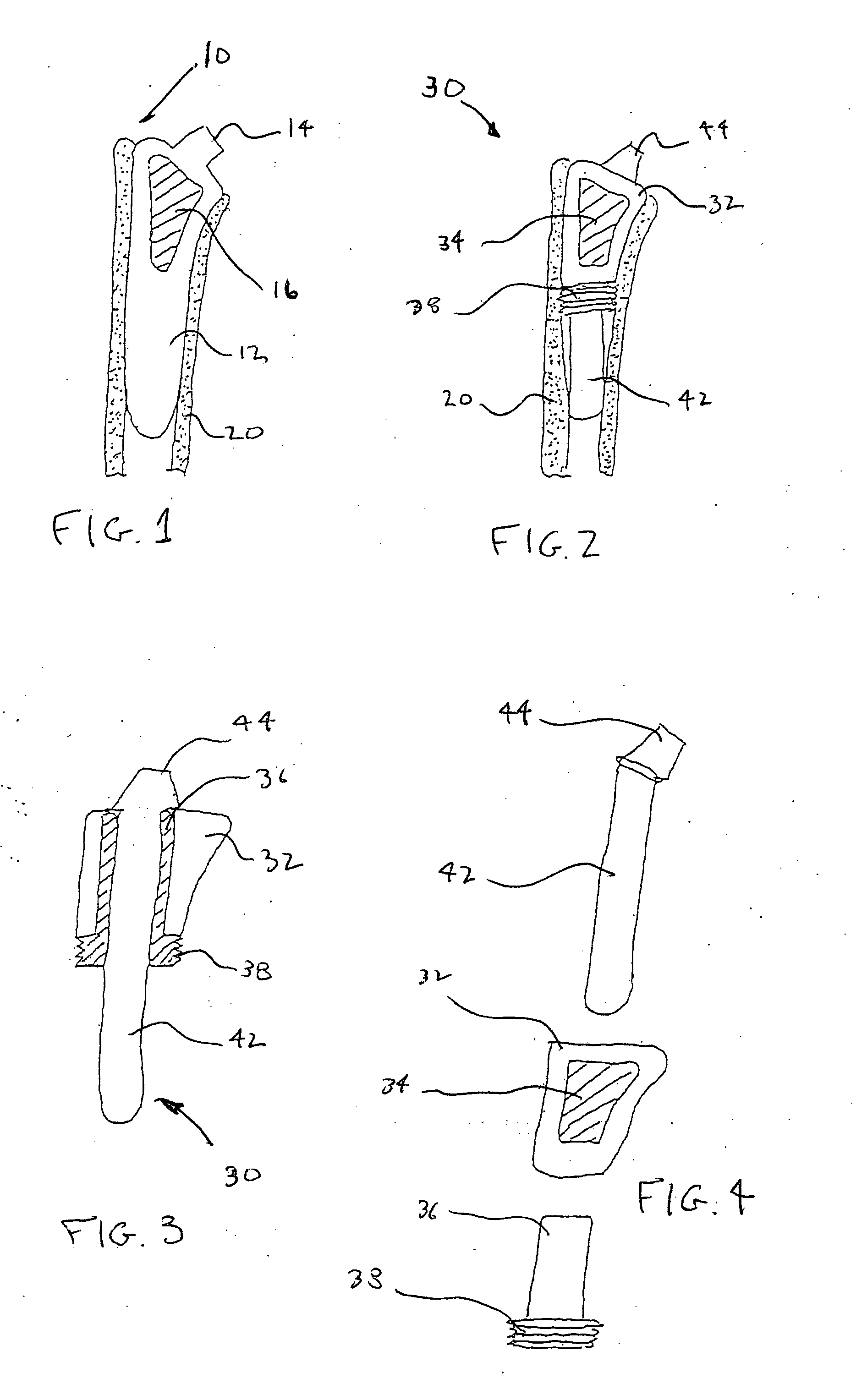
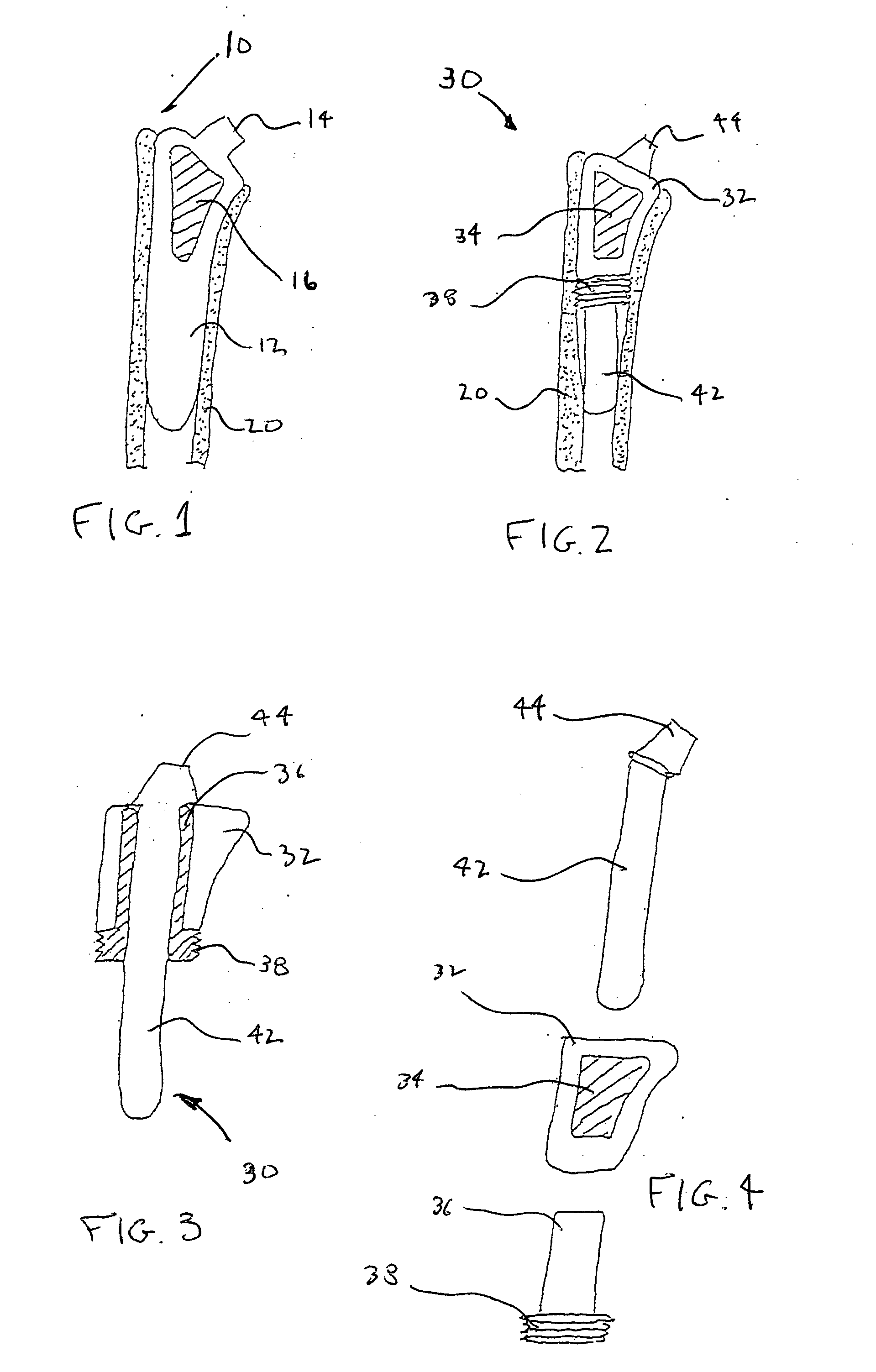
Methods and apparatus for performing therapeutic procedures in the spine
Methods and apparatus for forming one or more trans-sacral axial instrumentation / fusion (TASIF) axial bore through vertebral bodies in general alignment with a visualized, anterior or posterior axial instrumentation / fusion line (AAIFL or PAIFL) in a minimally invasive, low trauma, manner and providing a therapy to the spine employing the axial bore. Anterior or posterior starting positions aligned with the AAIFL or PAIFL are accessed through respective anterior and posterior tracts. Curved or relatively straight anterior and curved posterior TASIF axial bores are formed from the anterior and posterior starting positions. The therapies performed through the TASIF axial bores include discoscopy, full and partial discectomy, vertebroplasty, balloon-assisted vertebroplasty, drug delivery, electrical stimulation and various forms of spinal disc cavity augmentation, spinal disc replacement, fusion of spinal motion segments and implantation of radioactive seeds. Axial spinal implants and bone growth materials can be placed into single or multiple parallel or diverging TASIF axial bores to fuse two or more vertebrae, or distract or shock absorb two or more vertebrae.
Owner:MIS IP HLDG LLC
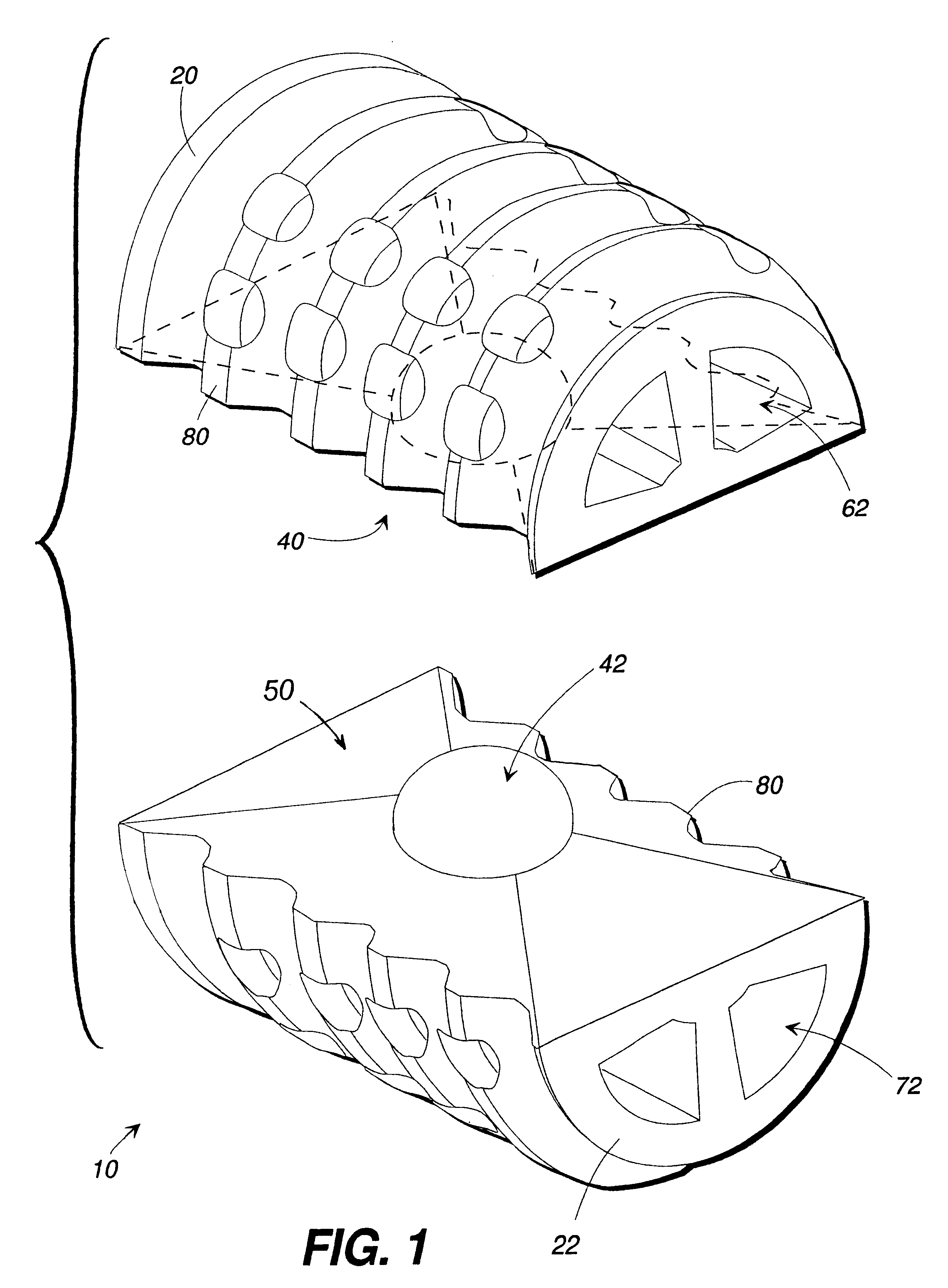
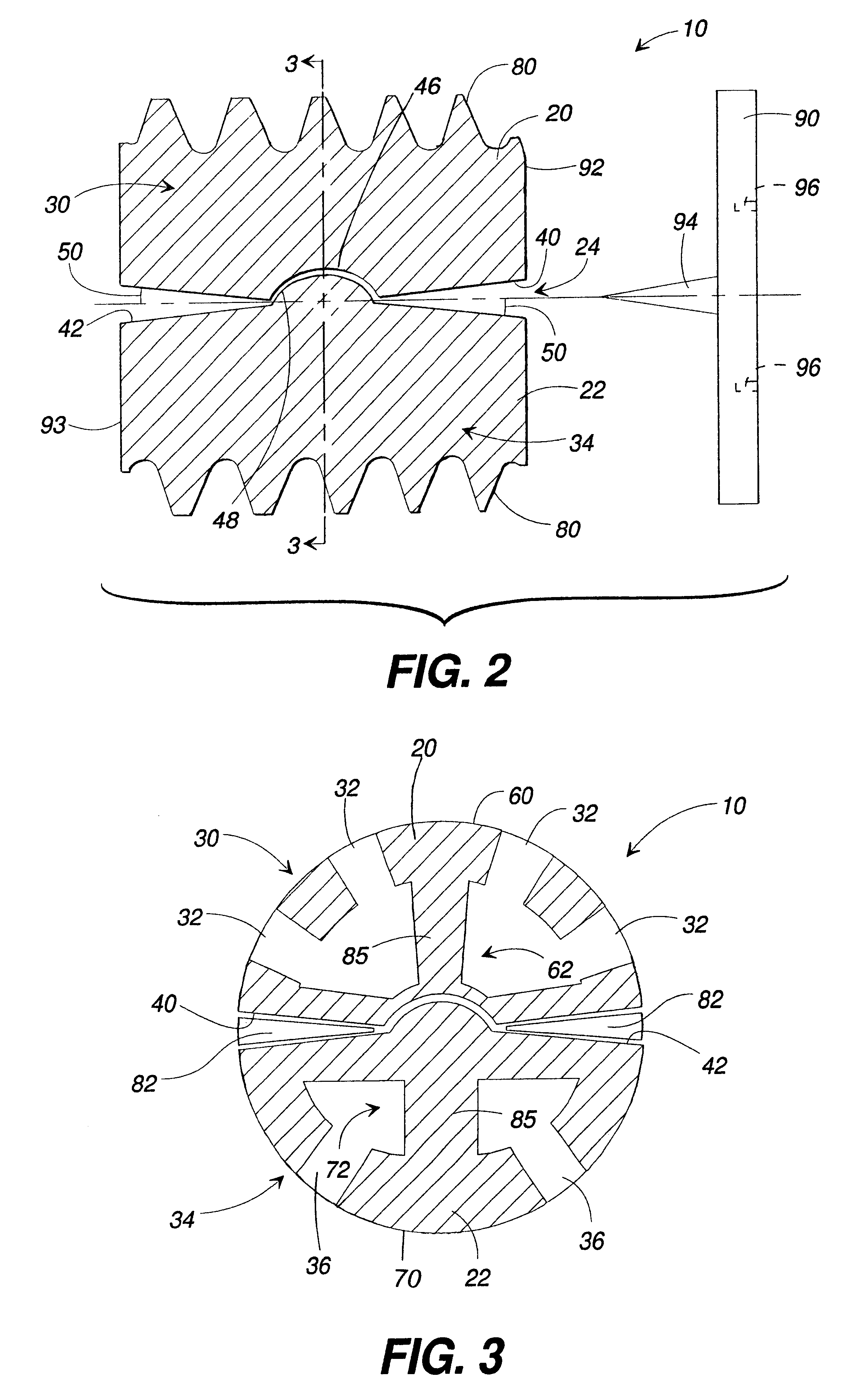
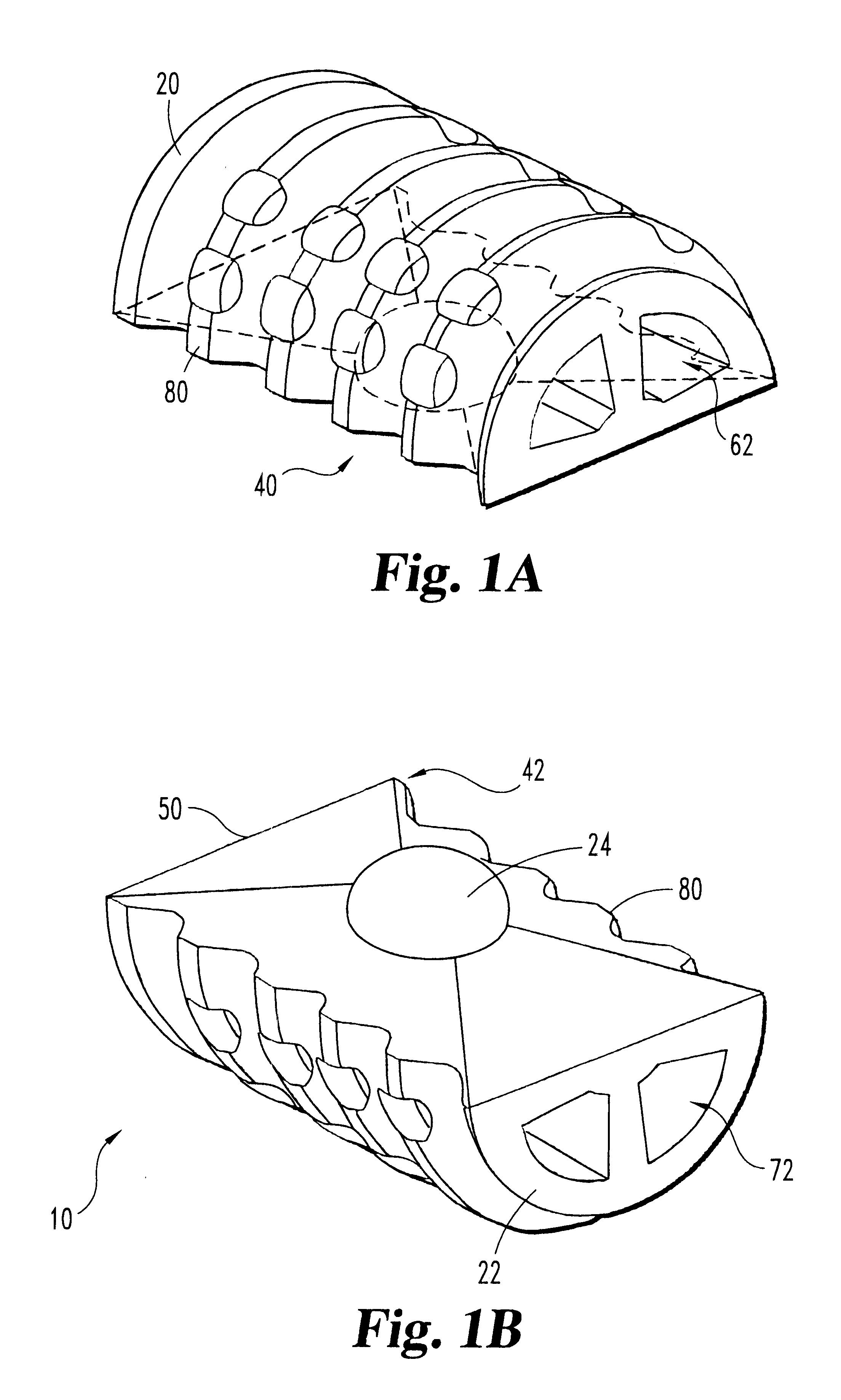
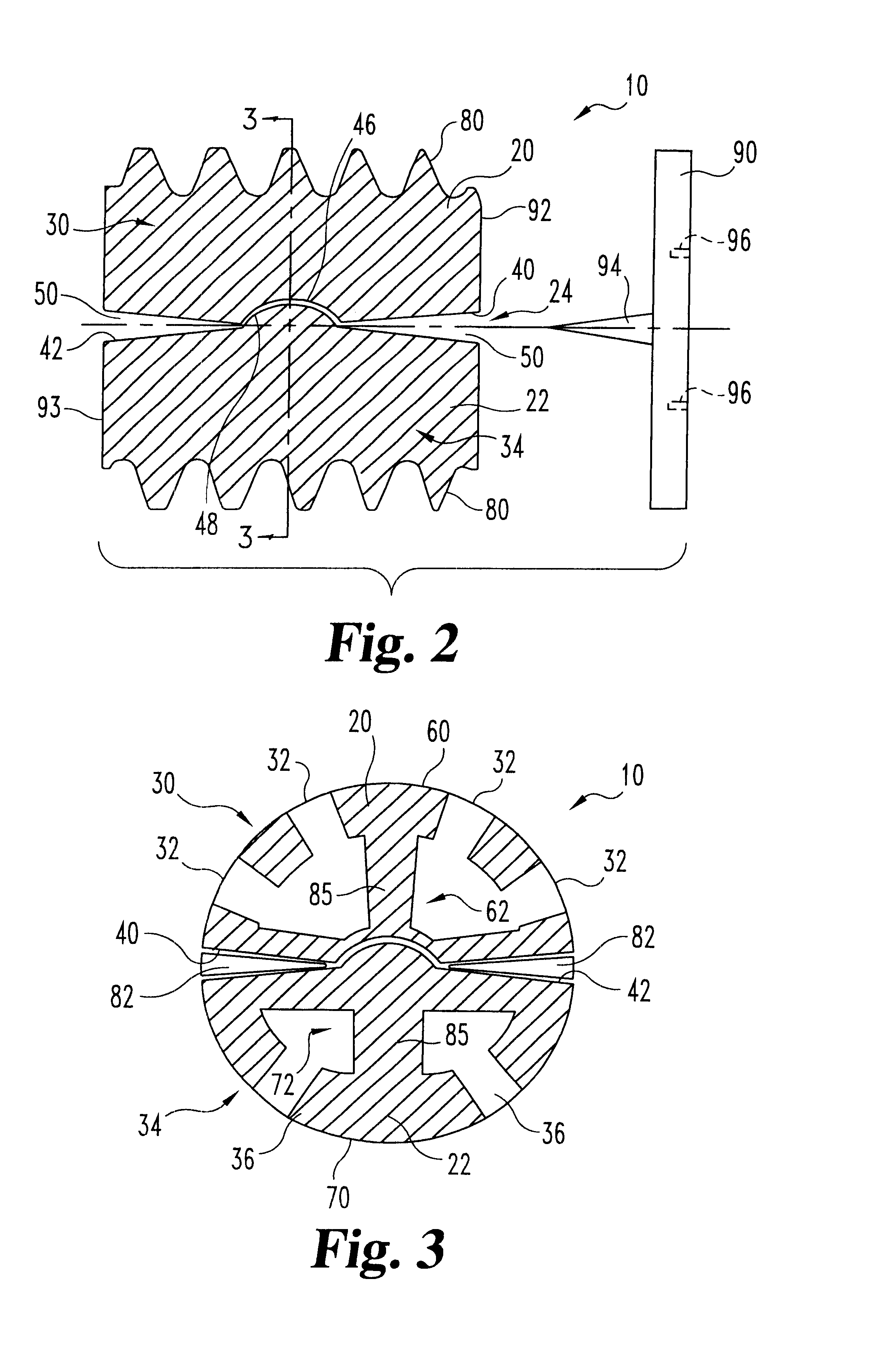
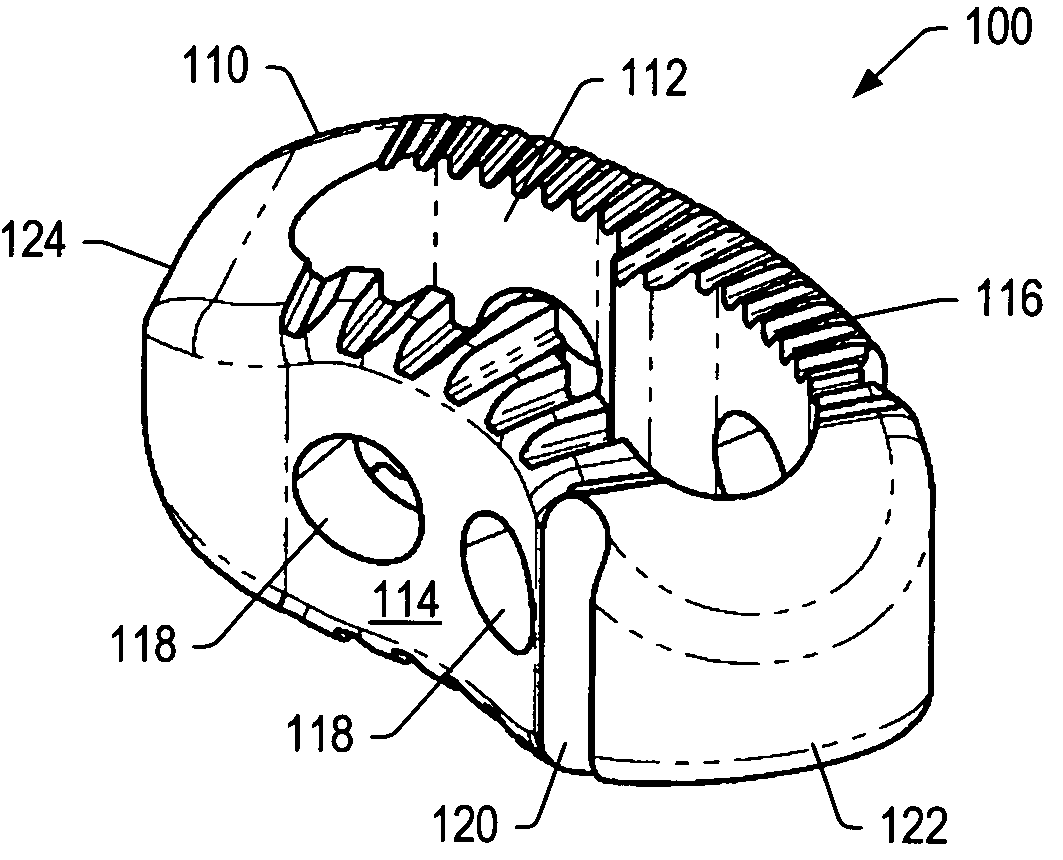
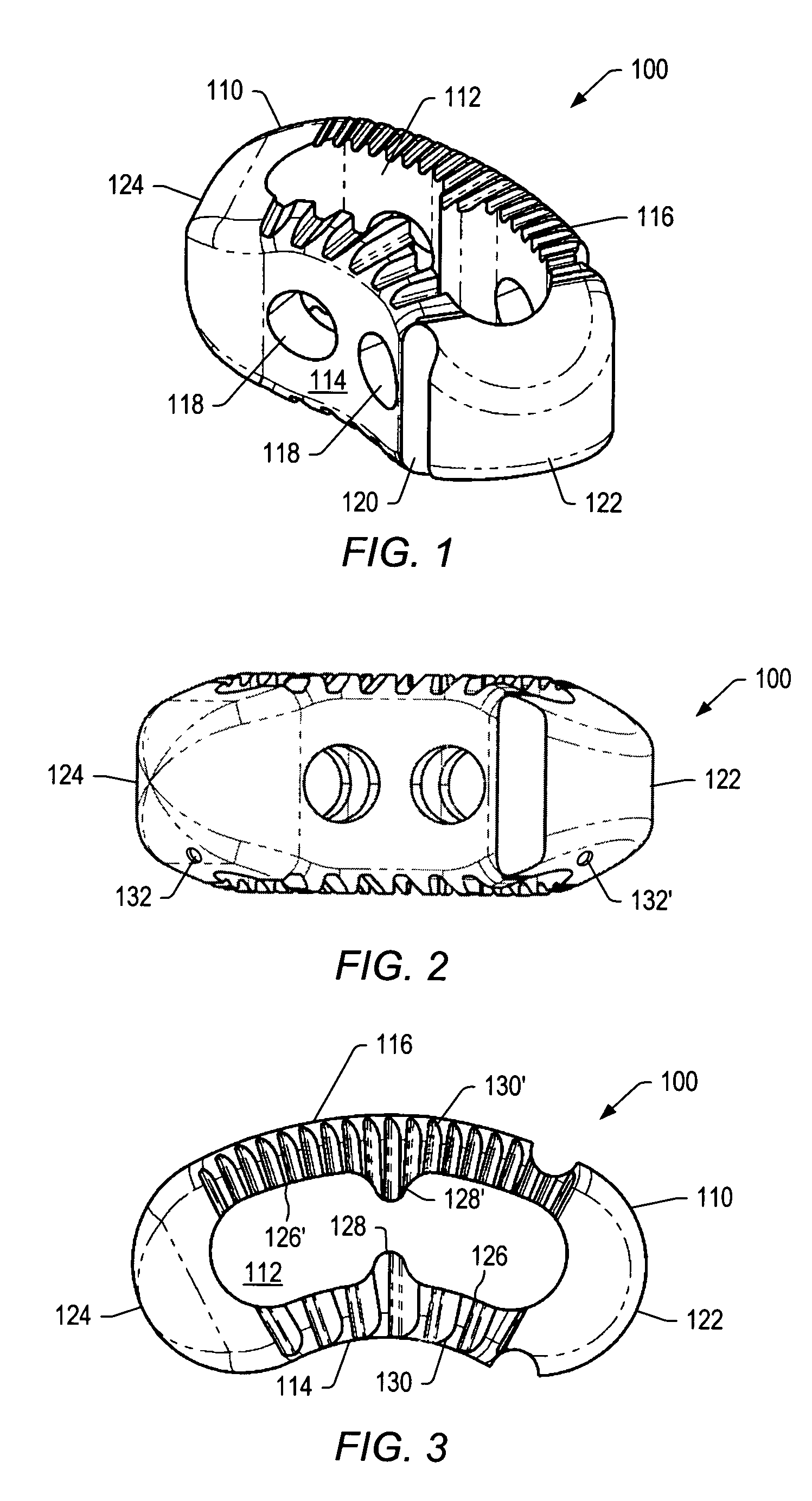
Articulating spinal implant
A spinal implant for intervertebral disc replacement. The implant is formed from two hemicylindrical elements, each engaging one of an adjacent pair of vertebrae. An articulating ball-and-socket joint between the two elements resists compression and lateral movement between the vertebra, but allows pivotal movement, thereby preserving mobility. Fusion chambers are provided for allowing bone ingrowth to fuse the elements to the vertebrae. Biocompatible, bioreabsorbable struts, shims, fillers and / or end caps are provided for temporary stabilization of the first and second hemicylindrical elements. Bone chips removed from the vertebrae during implantation or bone growth stimulators can be inserted into the fusion chamber or otherwise applied to the implant to enhance bone ingrowth.
Owner:WARSAW ORTHOPEDIC INC
Bone implants and methods
Implants, instruments and methods for bone fusion procedures are disclosed. In some embodiments, the implants are particularly advantageous for use between opposing vertebral bodies to facilitate stabilization or arthrodesis of an intervertebral joint. The implants include, at least, a support component that provides structural support during fusion. In a typical embodiment, the implants also include a growth component. A growth component provides an environment conducive to new bone growth between the bones being fused. Several unique configurations to enhance fusion, instruments for insertion and methods for insertion are also disclosed.
Owner:ZIMMER SPINE INC
Spinal implant
ActiveUS20050027360A1Easy to integratePromote bone growthDiagnosticsBone implantRaspIntervertebral disk
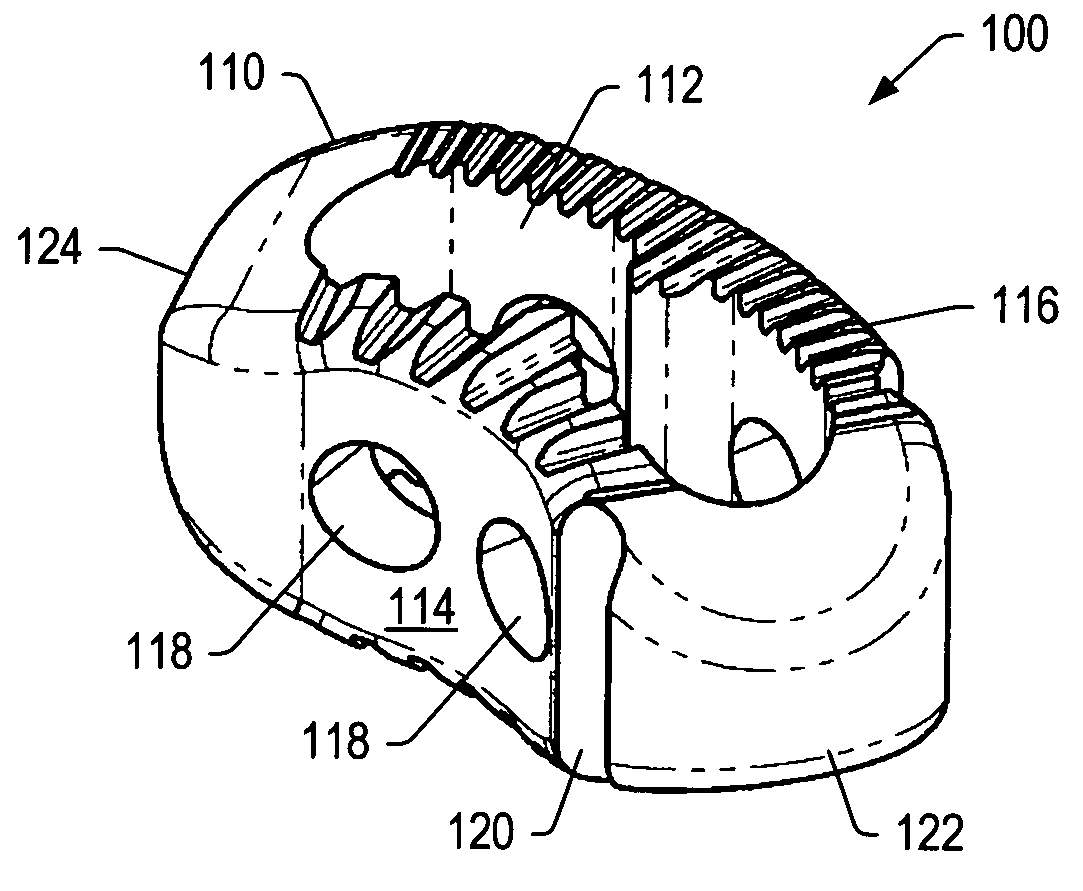
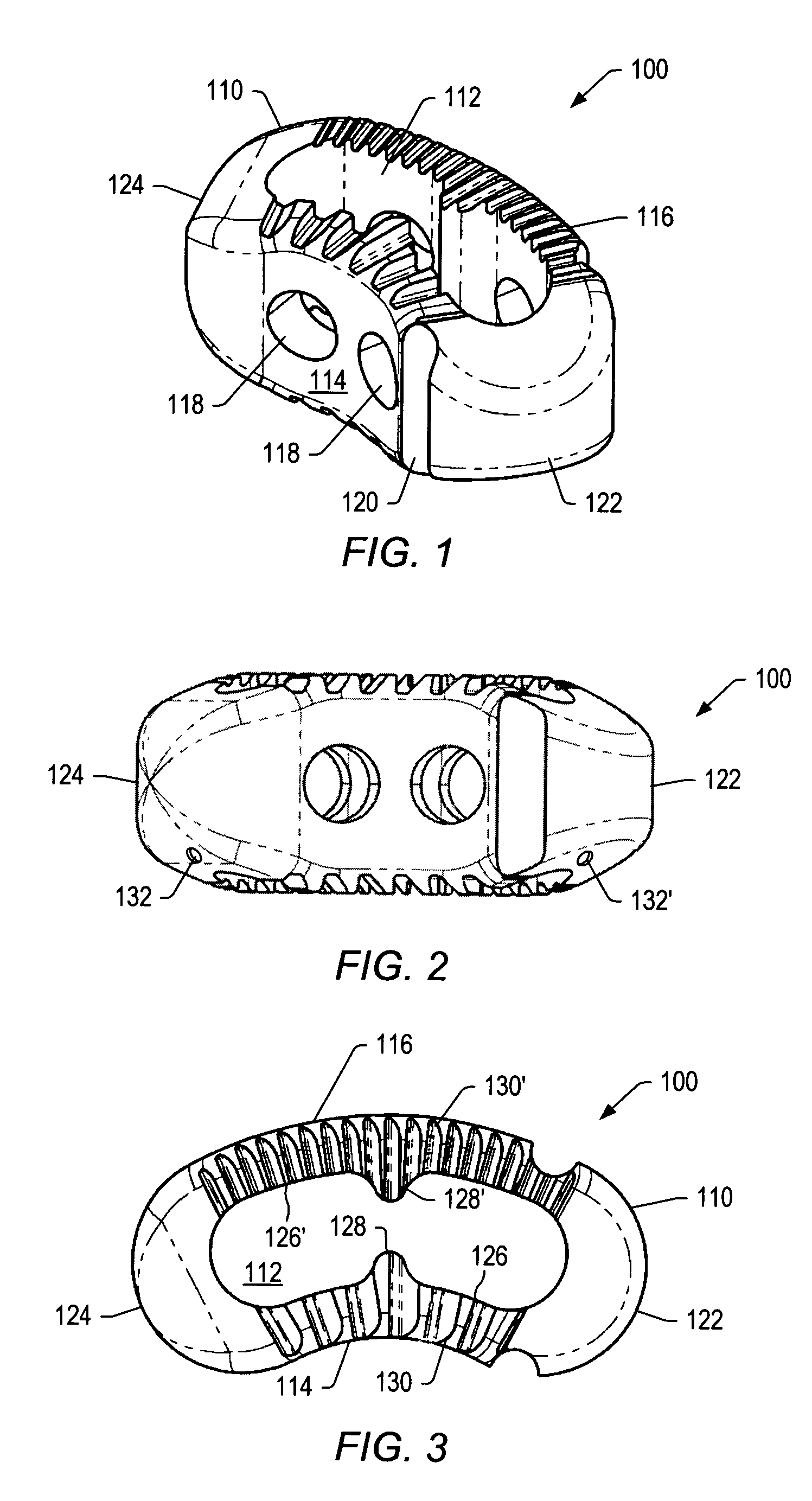
A spinal implant may be used to stabilize a portion of a spine. The implant may promote bone growth between adjacent vertebrae that fuses the vertebrae together. An implant may include an opening through a height of a body of the implant. The body of the implant may include curved sides. A top and / or a bottom of the implant may include protrusions that contact and / or engage vertebral surfaces to prevent backout of the implant from the disc space. A variety of instruments may be used to prepare a disc space and insert an implant. The instruments may include, but are not limited to, a distractor, a rasp, and one or more guides. The implant and instruments may be supplied in an instrument kit.
Owner:ZIMMER BIOMET SPINE INC
Articulating spinal implant
A spinal implant for intervertebral disc replacement. The implant is formed from two hemicylindrical elements, each engaging one of an adjacent pair of vertebrae. An articulating ball-and-socket joint and / or rocker and channel between the two elements resists compression and lateral movement between the vertebra, but allows pivotal movement, thereby preserving mobility. Fusion chambers are provided for allowing bone ingrowth to fuse the elements to the vertebrae. Biocompatible, bioreabsorbable struts, shims, fillers and / or end caps are provided for temporary stabilization of the first and second hemicylindrical elements. Bone chips removed from the vertebrae during implantation or bone growth stimulators can be inserted into the fusion chamber or otherwise applied to the implant to enhance bone ingrowth.
Owner:WARSAW ORTHOPEDIC INC
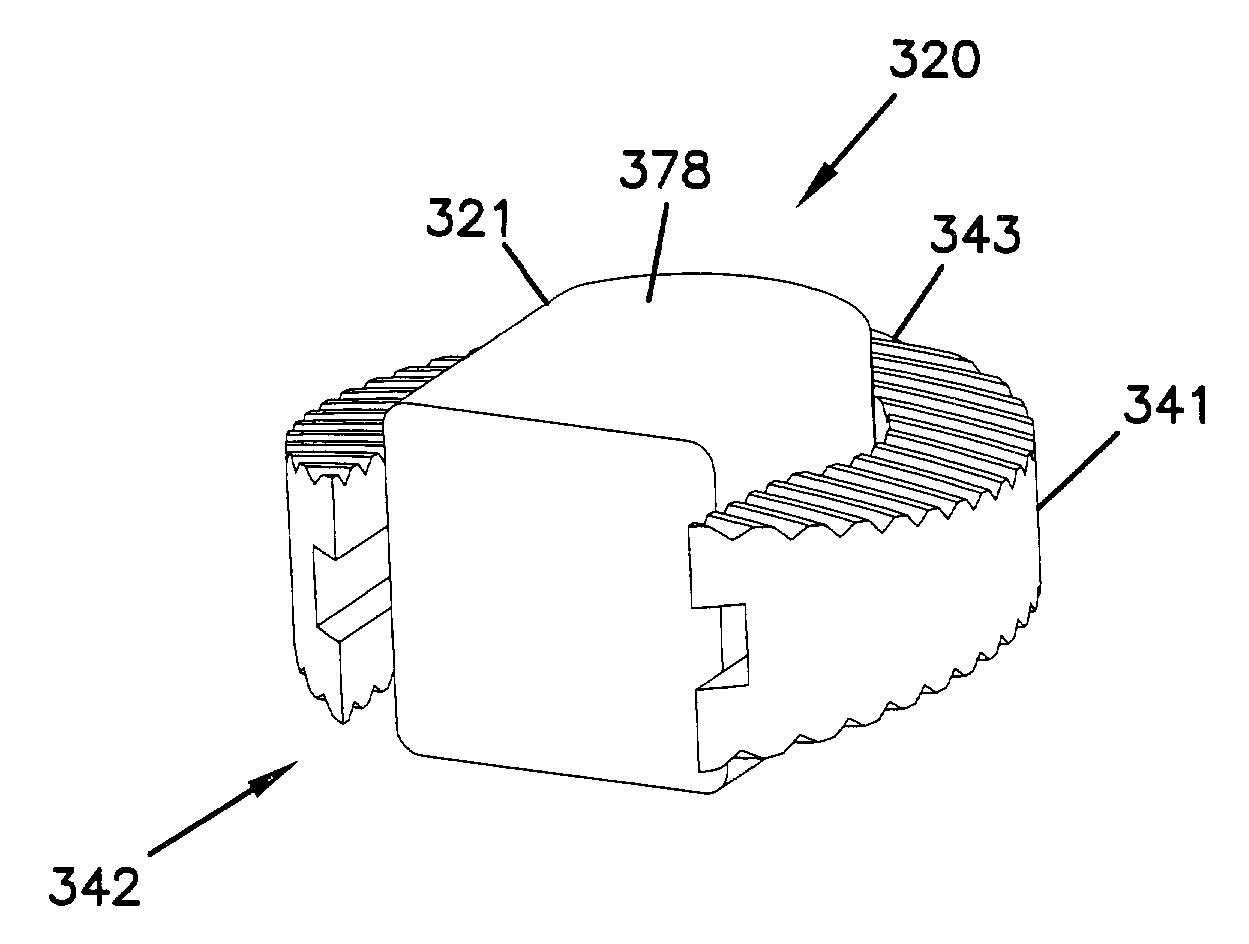
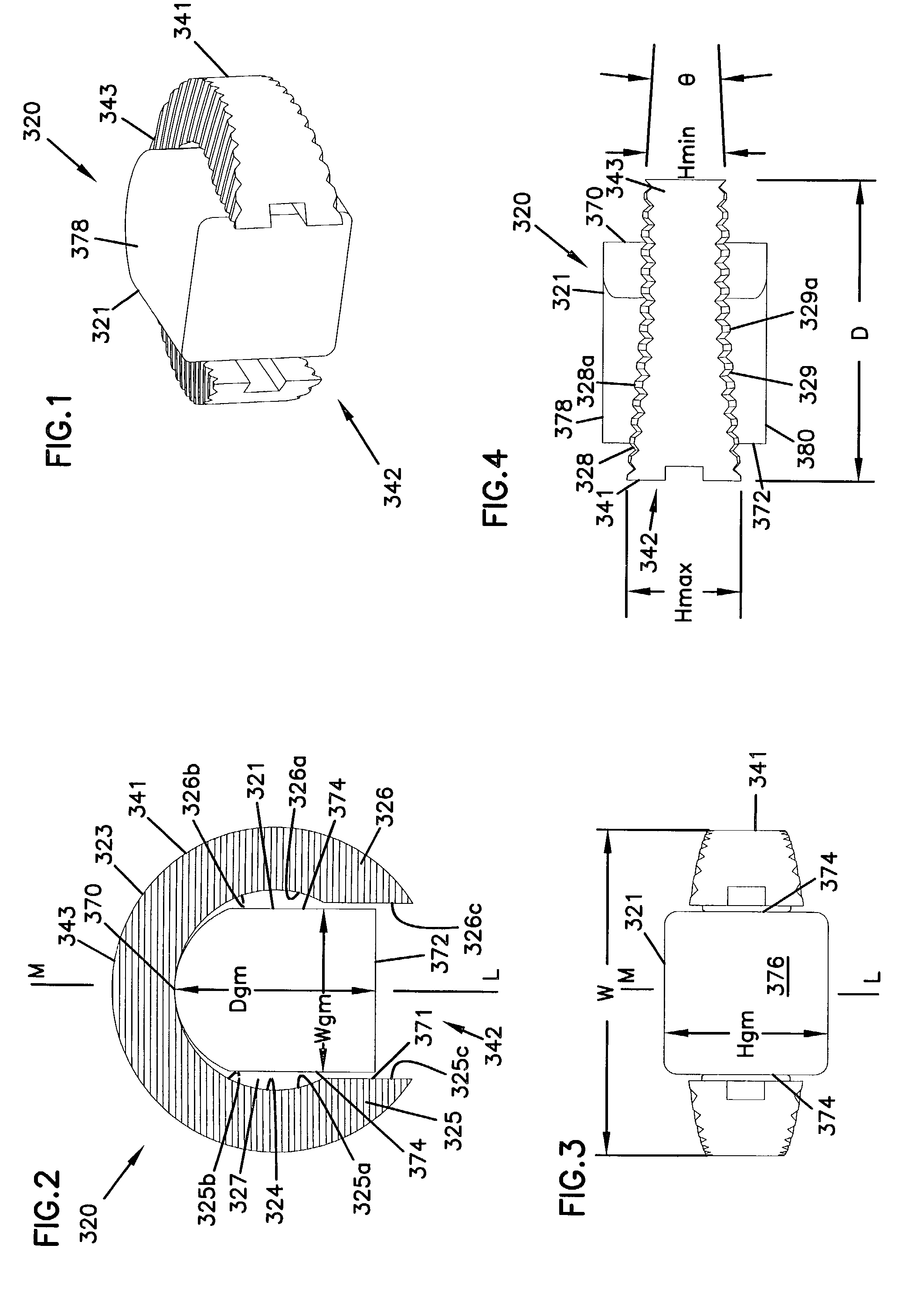
Expandable spinal implants
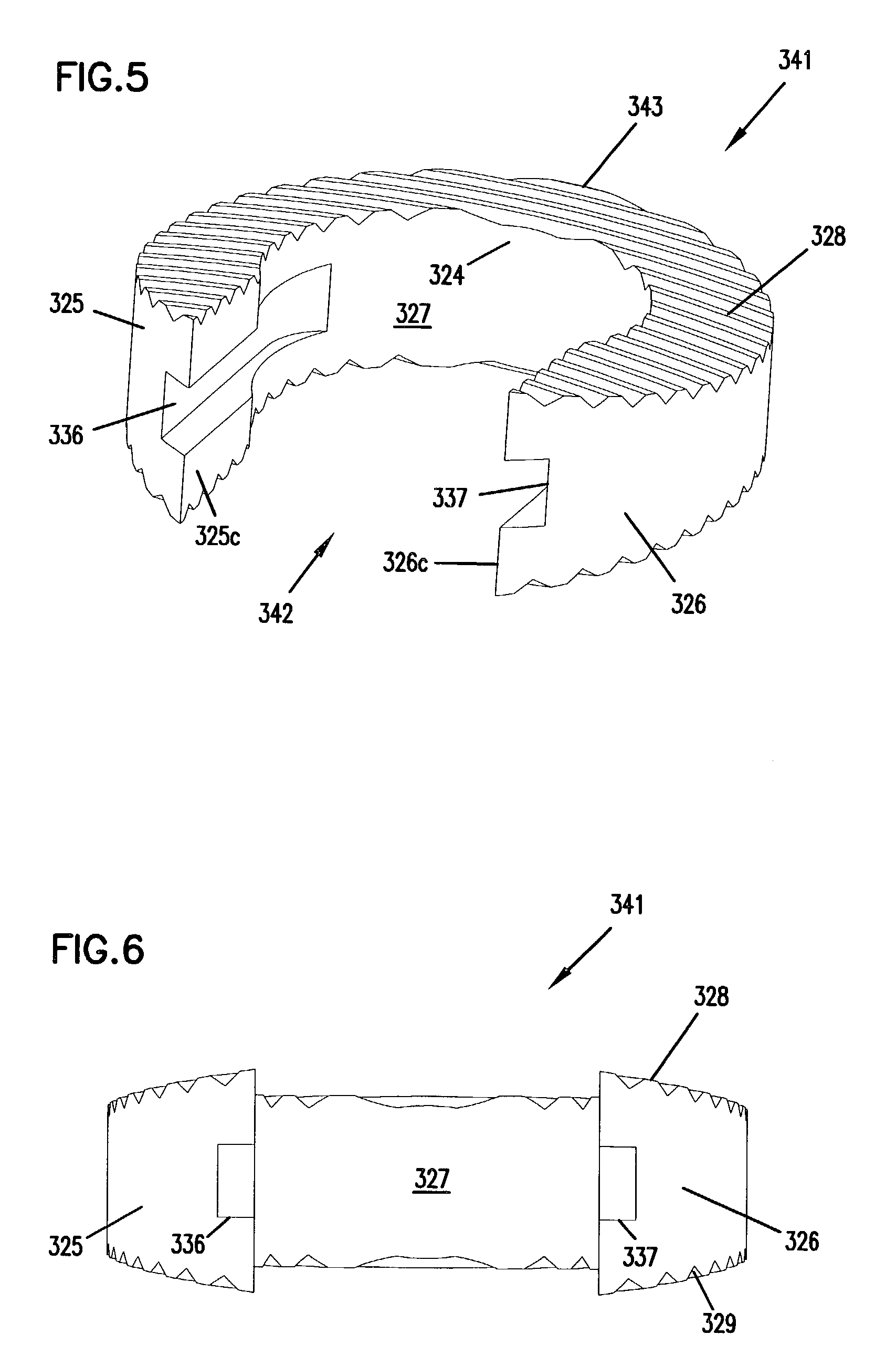
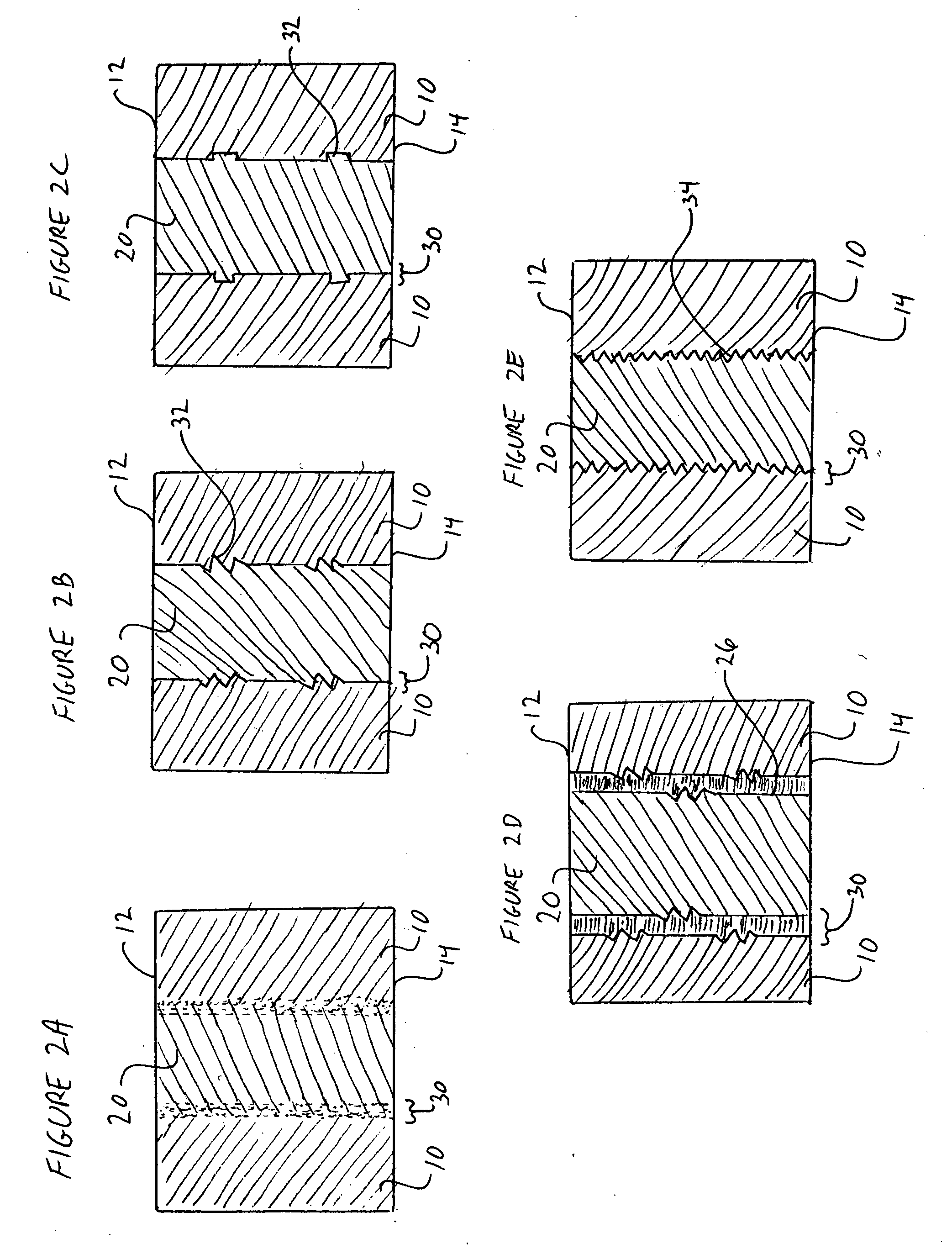
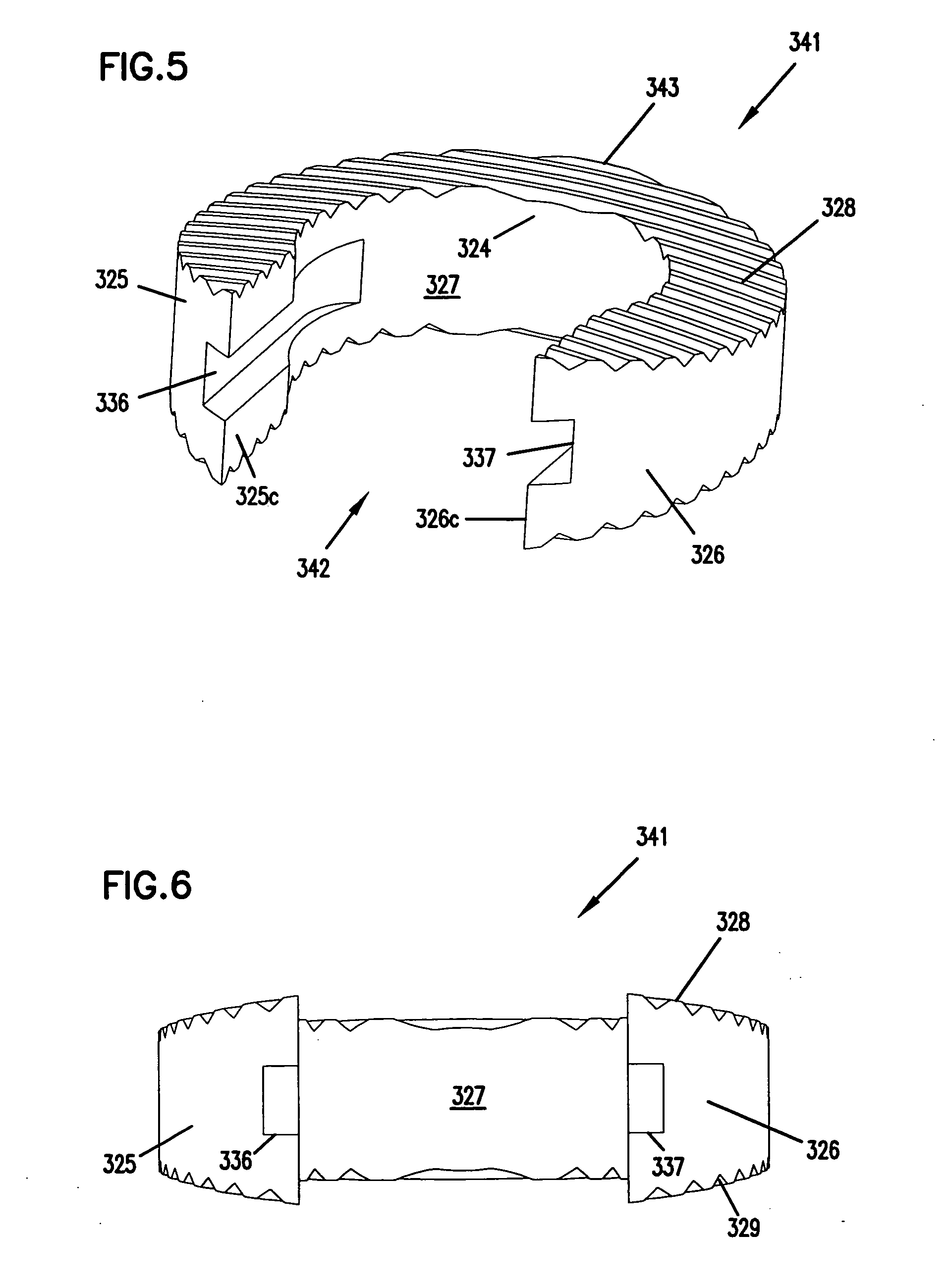
A cylinder-shaped expandable spinal implant is disclosed. The implant is a hollow housing having a cylinder-shaped wall, with a circular sealed distal end, and an opposite proximal circular open end. The open end of the housing has internal threads for securing a cap to it. A removable cap is threaded on the open end of the housing. The housing has two opposite large rectangular openings located longitudinally in the wall of the housing for receiving two arcuate sections. Each arcuate section is positioned in the rectangular opening in the wall of the implant. A locking means for locking the arcuate section in the rectangular opening in the wall is provided. A plurality of small ports are drilled in the wall for allowing bone growth after implantation of said implant in a patient. The arcuate sections have transverse ribs for locking the implant in position are implantation. Openings between the ribs are provided for allowing bone growth.
Owner:KOROS TIBOR
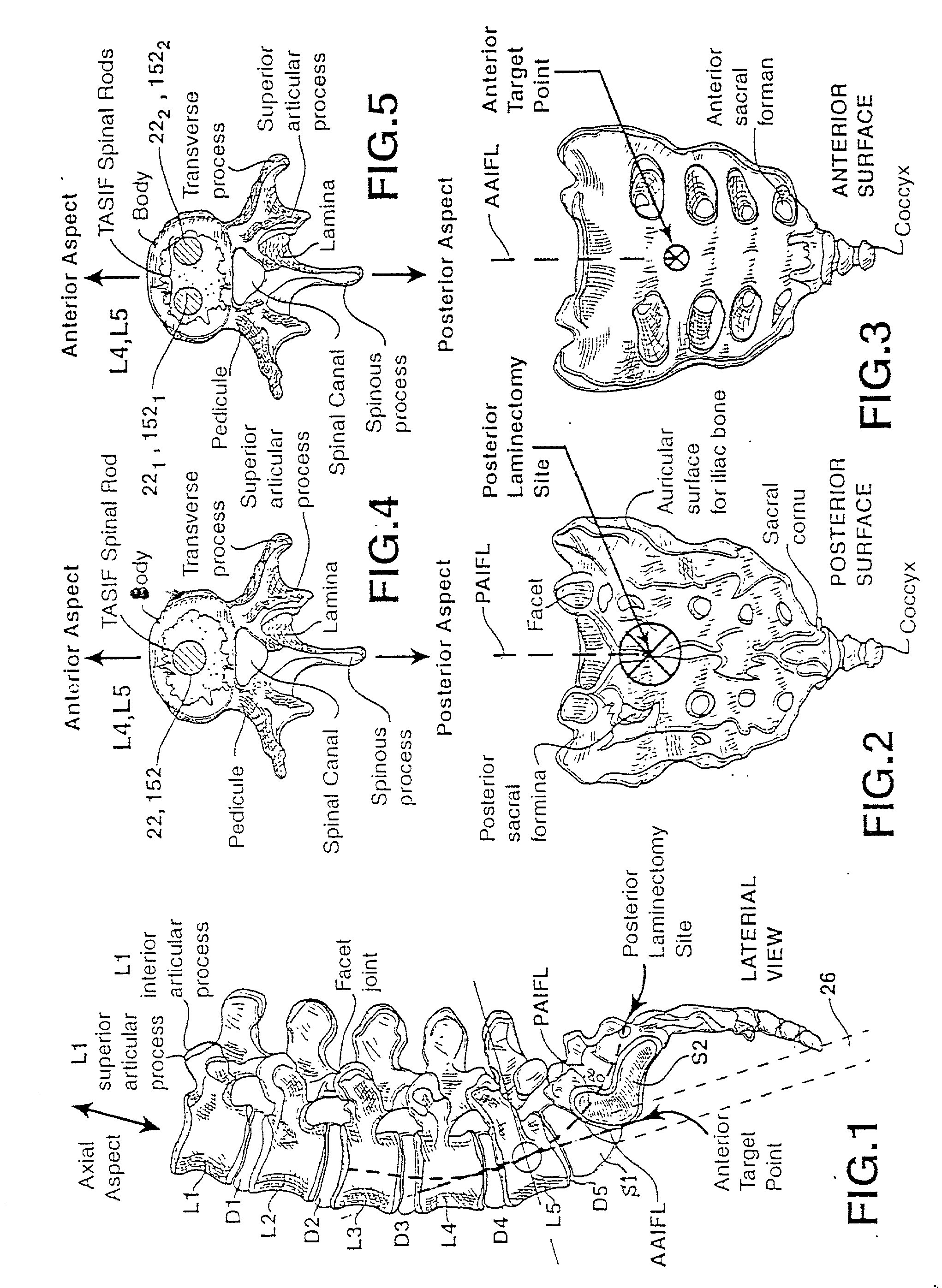
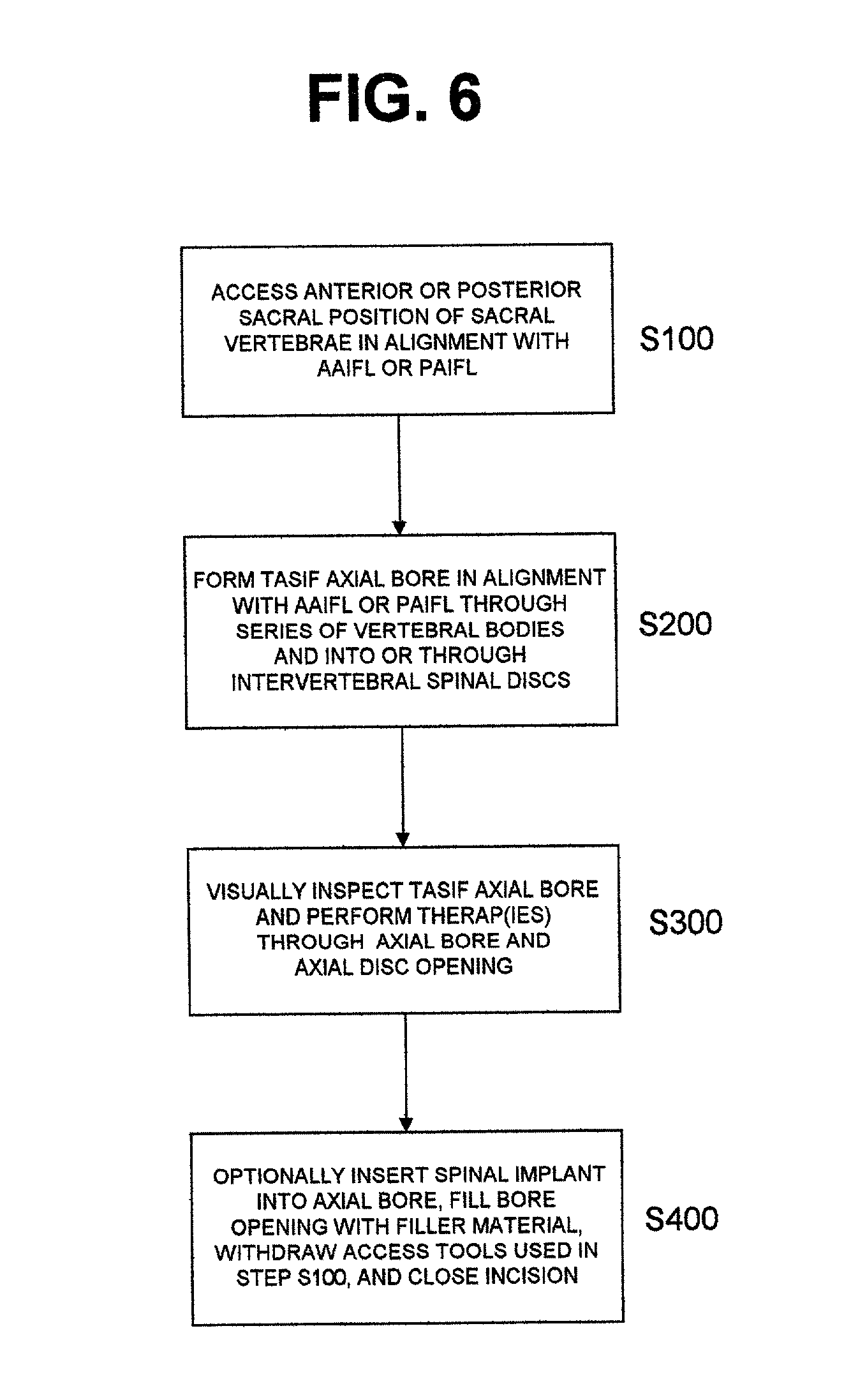
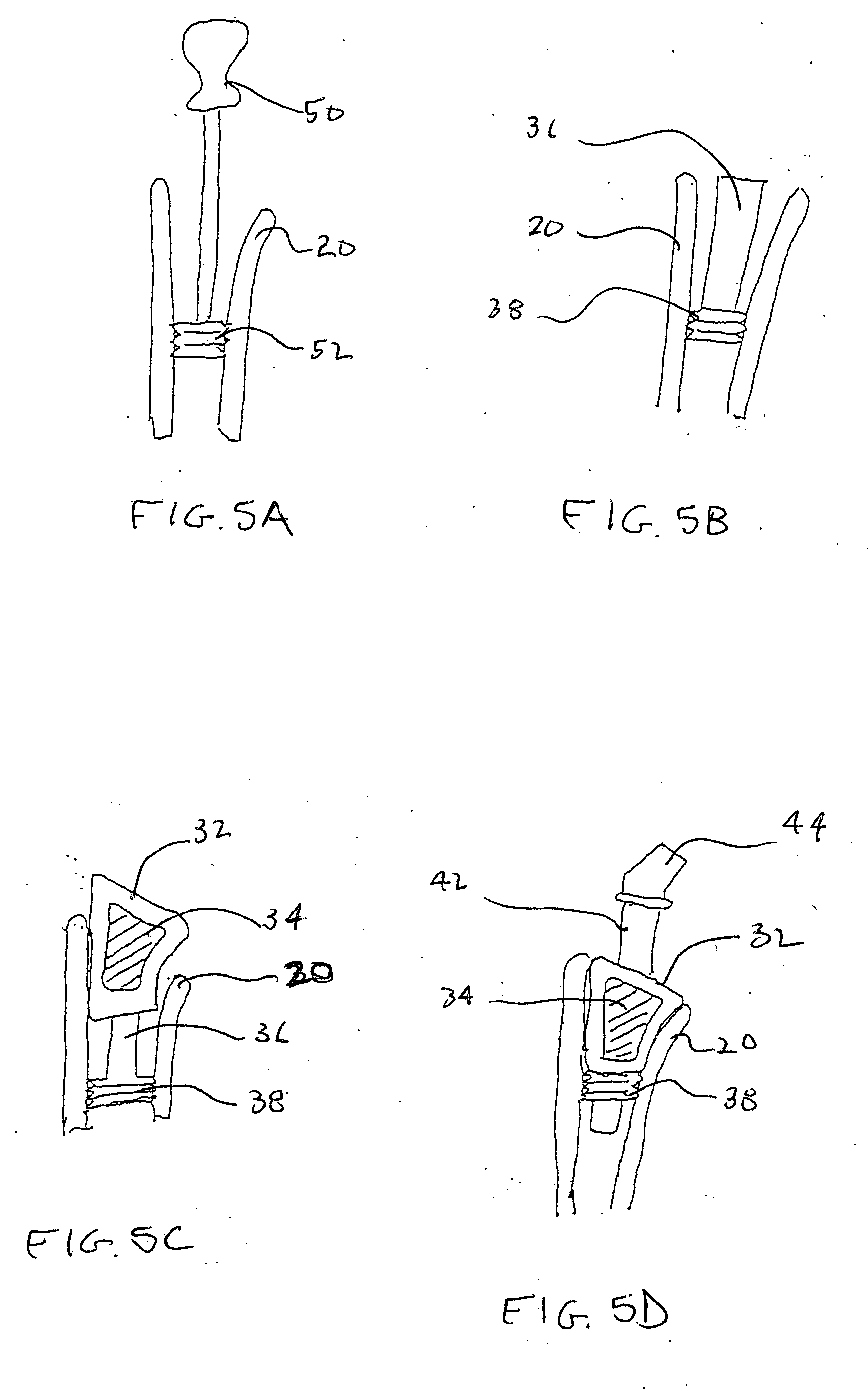
Methods and apparatus for performing therapeutic procedures in the spine
Methods and apparatus for forming one or more trans-sacral axial instrumentation / fusion (TASIF) axial bore through vertebral bodies in general alignment with a visualized, anterior or posterior axial instrumentation / fusion line (AAIFL or PAIFL) in a minimally invasive, low trauma, manner and providing a therapy to the spine employing the axial bore. Anterior or posterior starting positions aligned with the AAIFL or PAIFL are accessed through respective anterior and posterior tracts. Curved or relatively straight anterior and curved posterior TASIF axial bores are formed from the anterior and posterior starting positions. The therapies performed through the TASIF axial bores include discoscopy, full and partial discectomy, vertebroplasty, balloon-assisted vertebroplasty, drug delivery, electrical stimulation and various forms of spinal disc cavity augmentation, spinal disc replacement, fusion of spinal motion segments and implantation of radioactive seeds. Axial spinal implants and bone growth materials can be placed into single or multiple parallel or diverging TASIF axial bores to fuse two or more vertebrae, or distract or shock absorb two or more vertebrae.
Owner:MIS IP HLDG LLC
Malleable putty and flowable paste with allograft bone having residual calcium for filling bone defects
The invention is directed toward a malleable bone putty and a flowable pastel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of partially demineralized lyophilized allograft bone material having a residual calcium content ranging from 4 to 8% dry weight. The bone powder has a particle size ranging from about 100 to about 800 microns and is mixed in a high molecular weight hydrogel carrier containing a sodium phosphate saline buffer, the hydrogel component of the carrier ranging from about 1.00 to 50% of the composition and having a molecular weight of about at least 700,000 Daltons. The composition has a pH between 6.8-7.4 contains about 25% to about 35% bone powder and can be additionally provided with BMP's.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
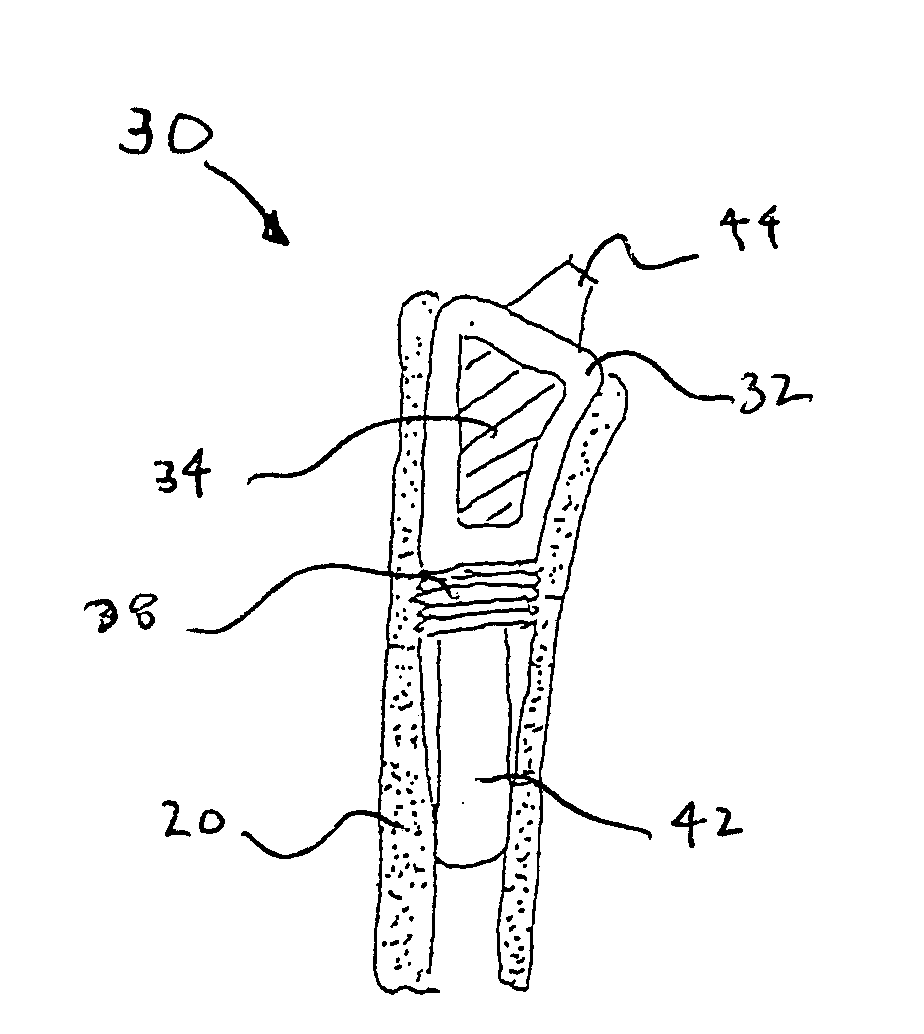
Methods and apparatus for performing therapeutic procedures in the spine
Owner:MIS IP HLDG LLC
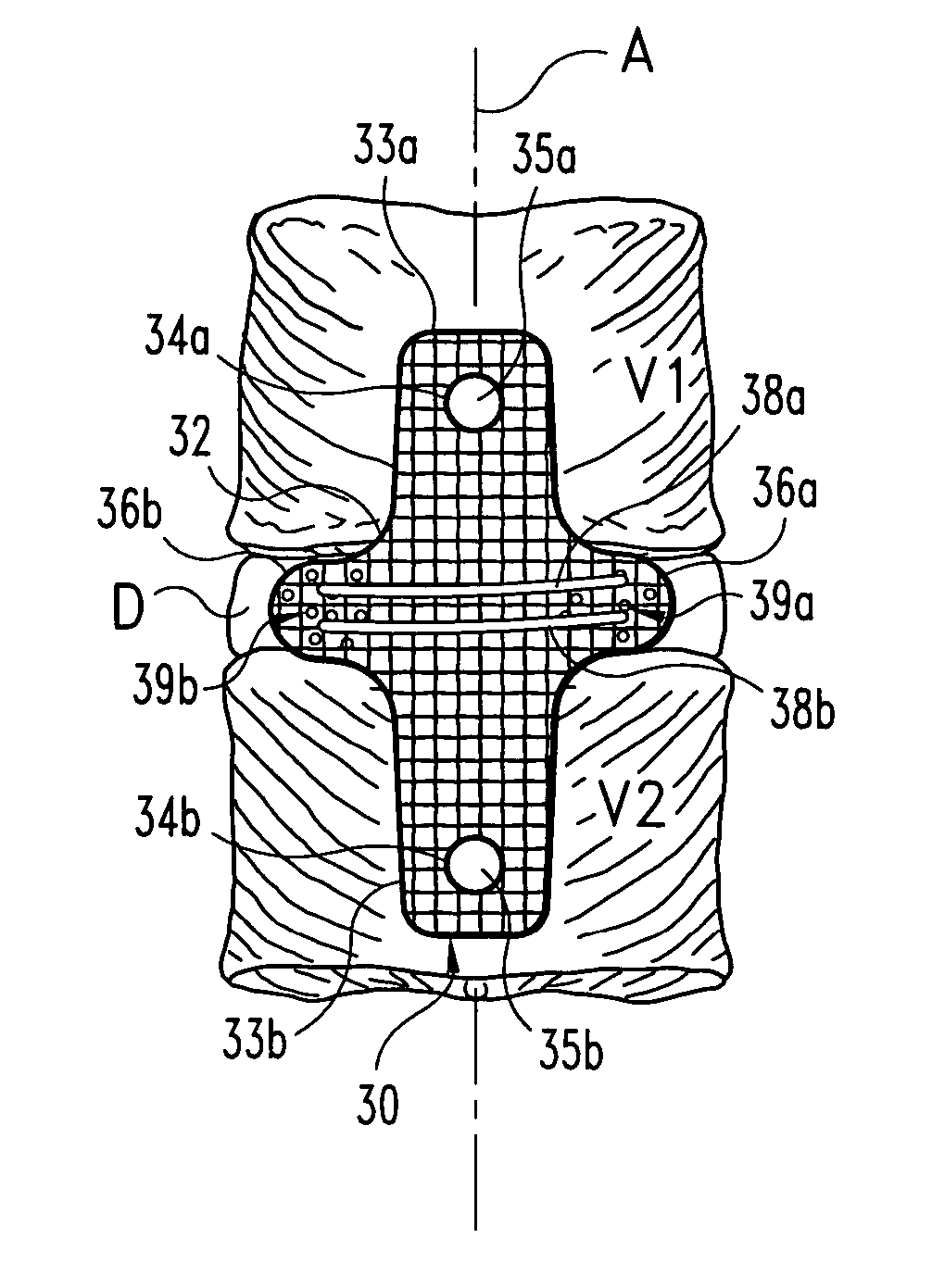
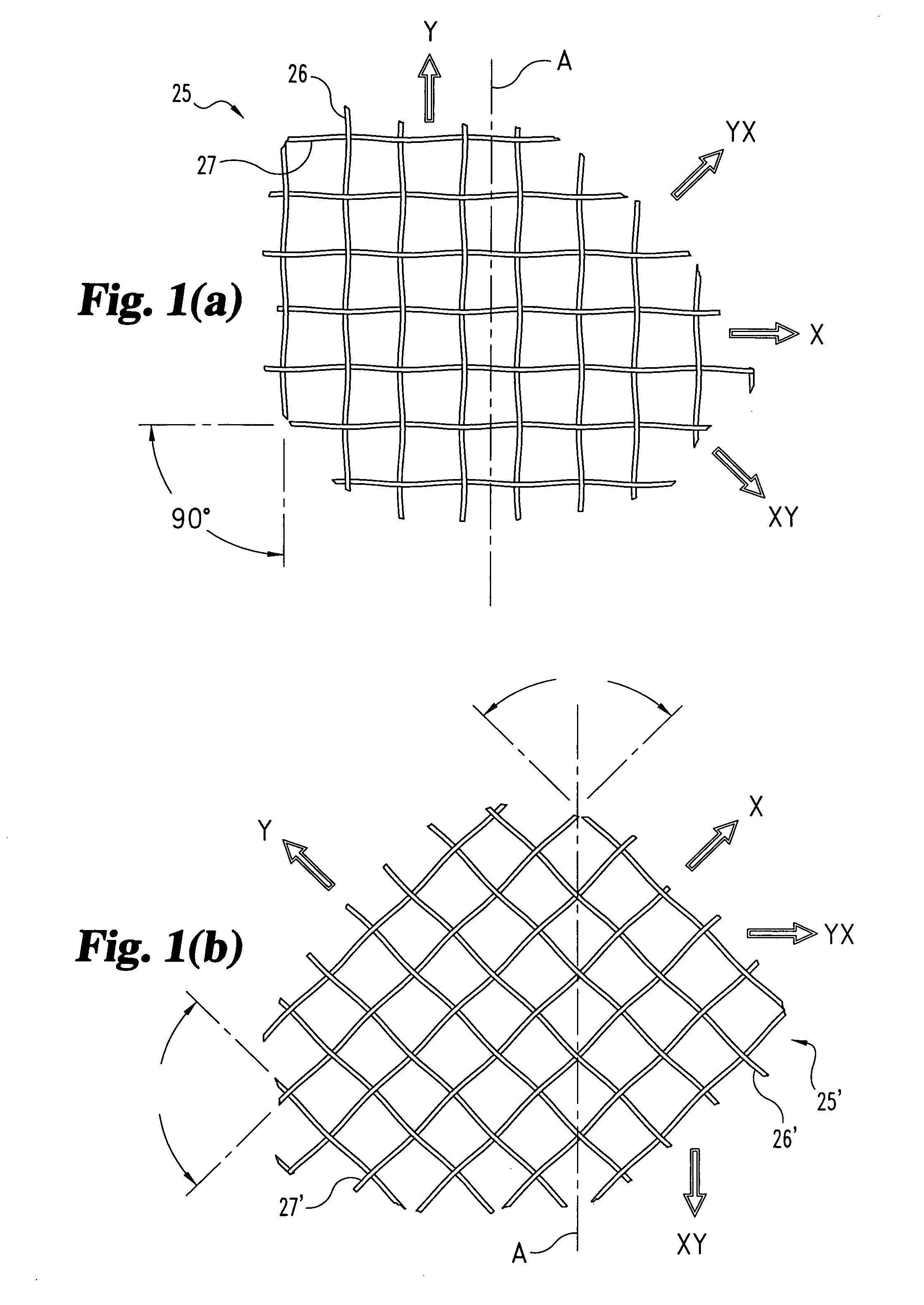
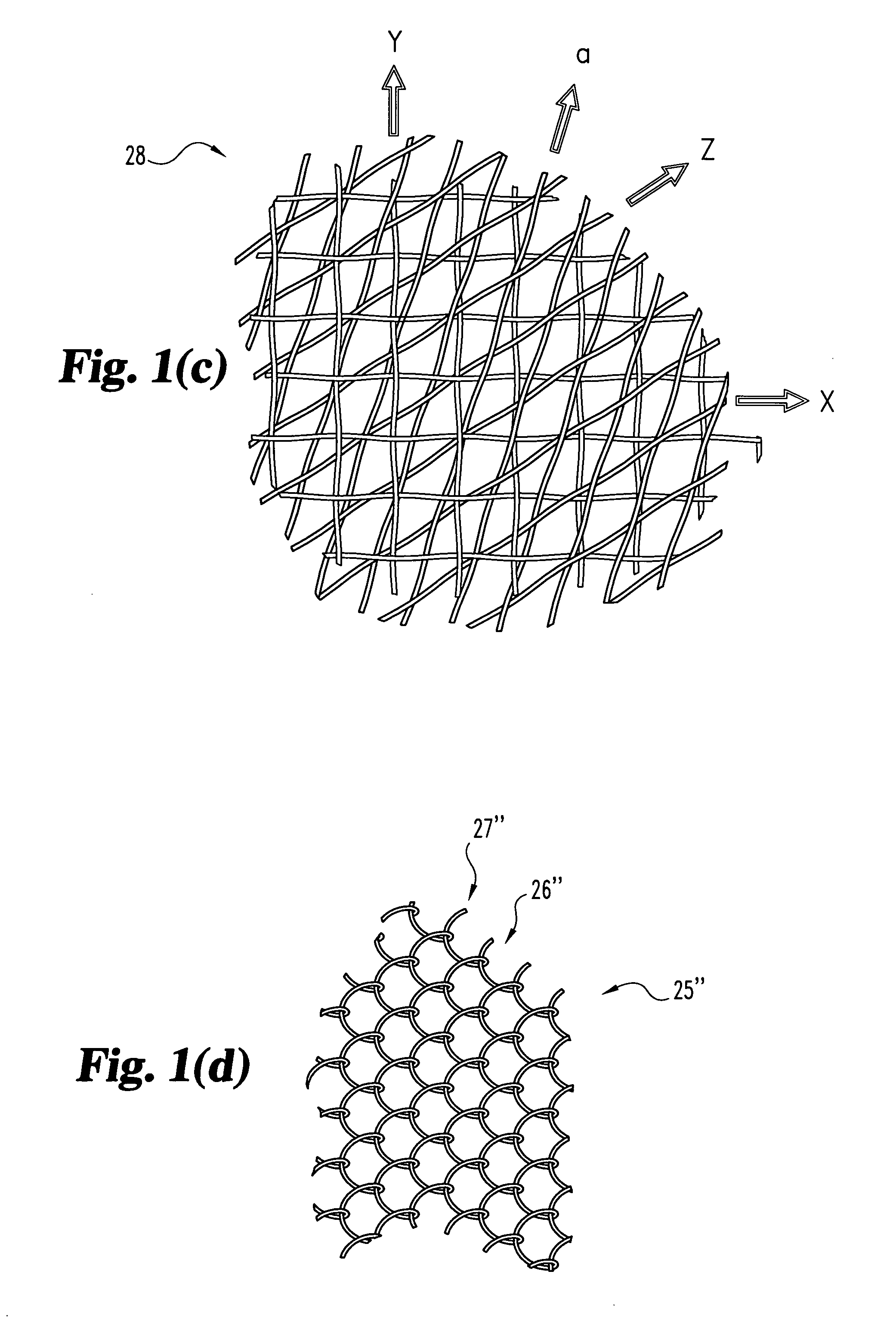
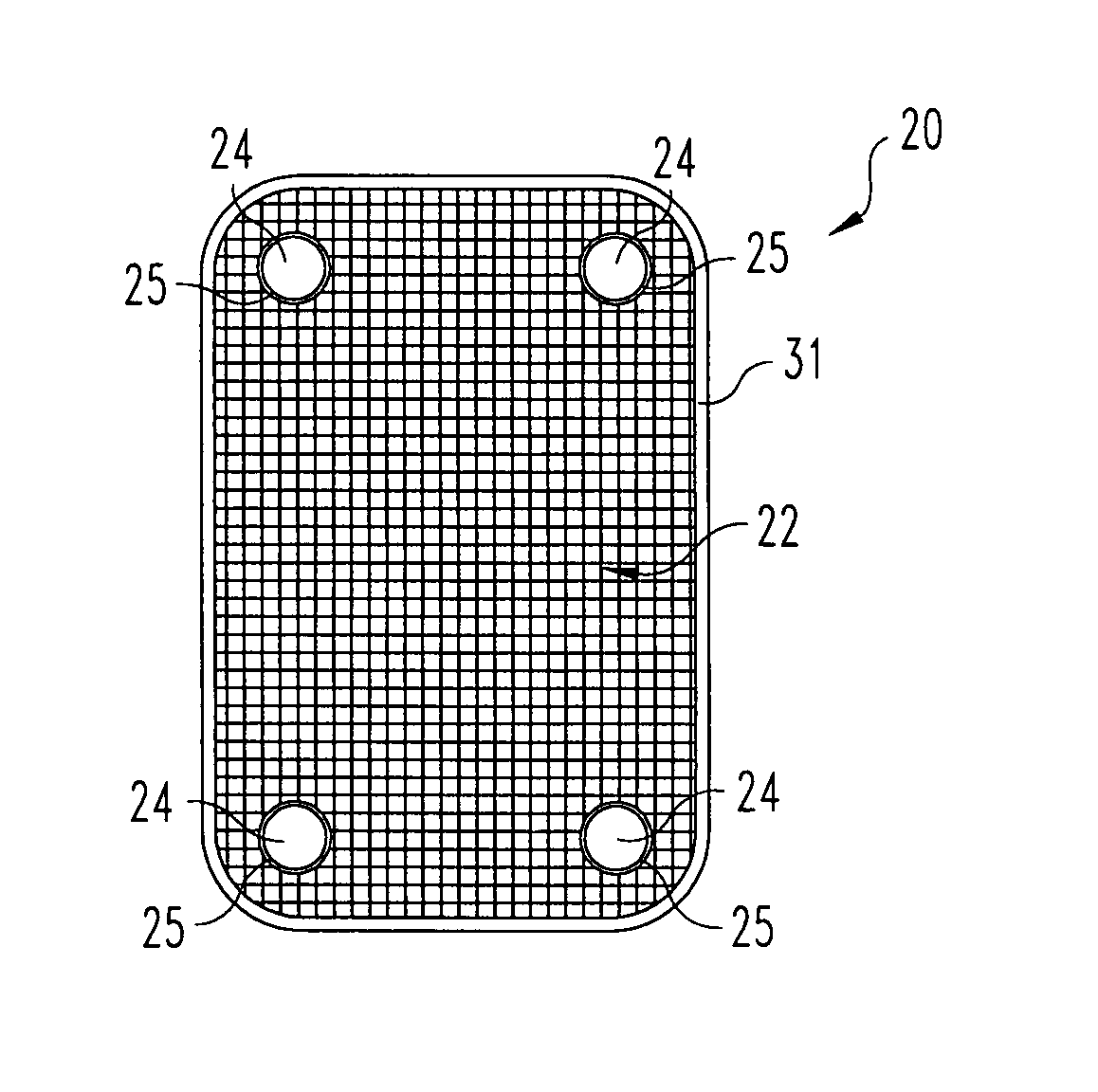
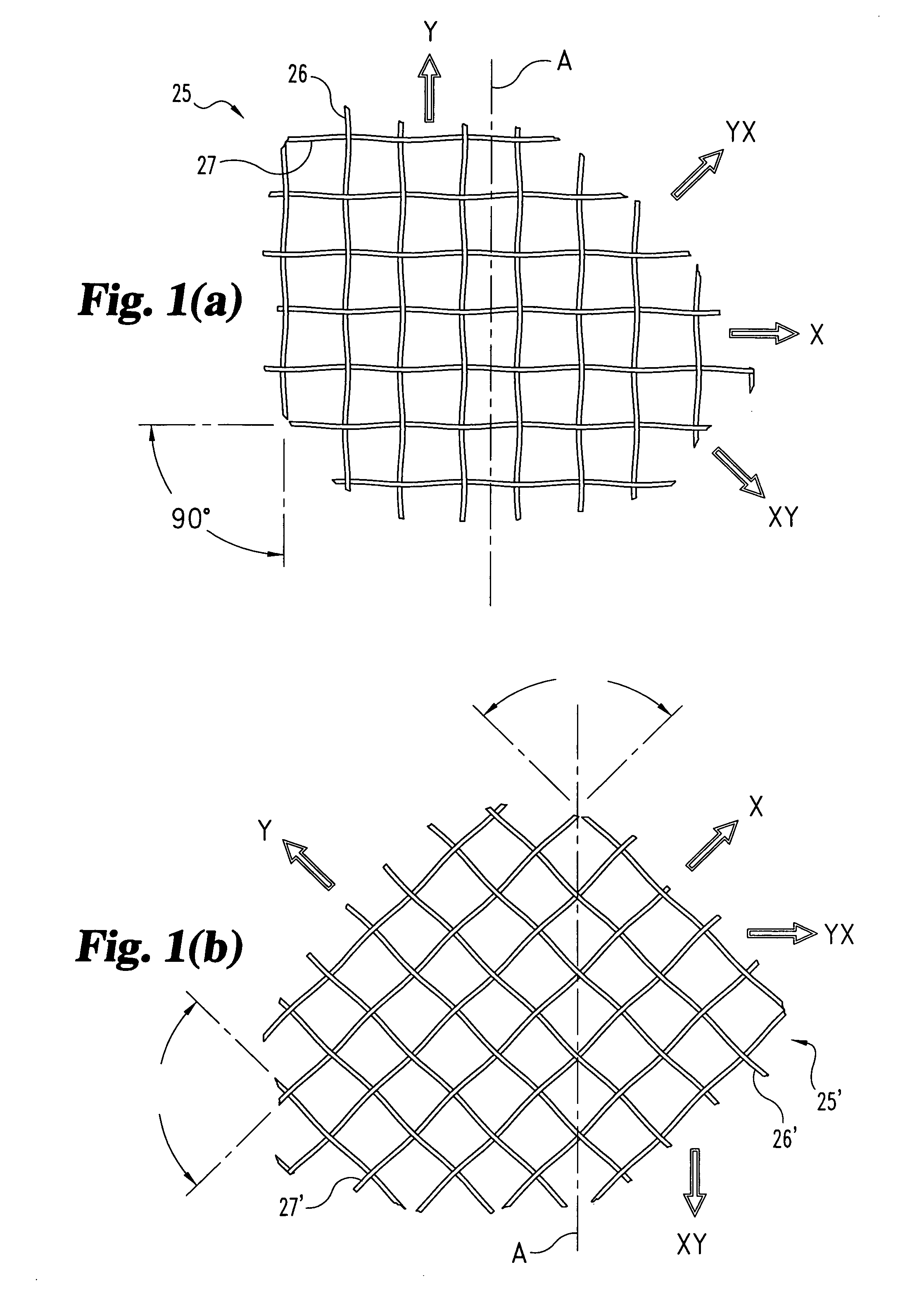
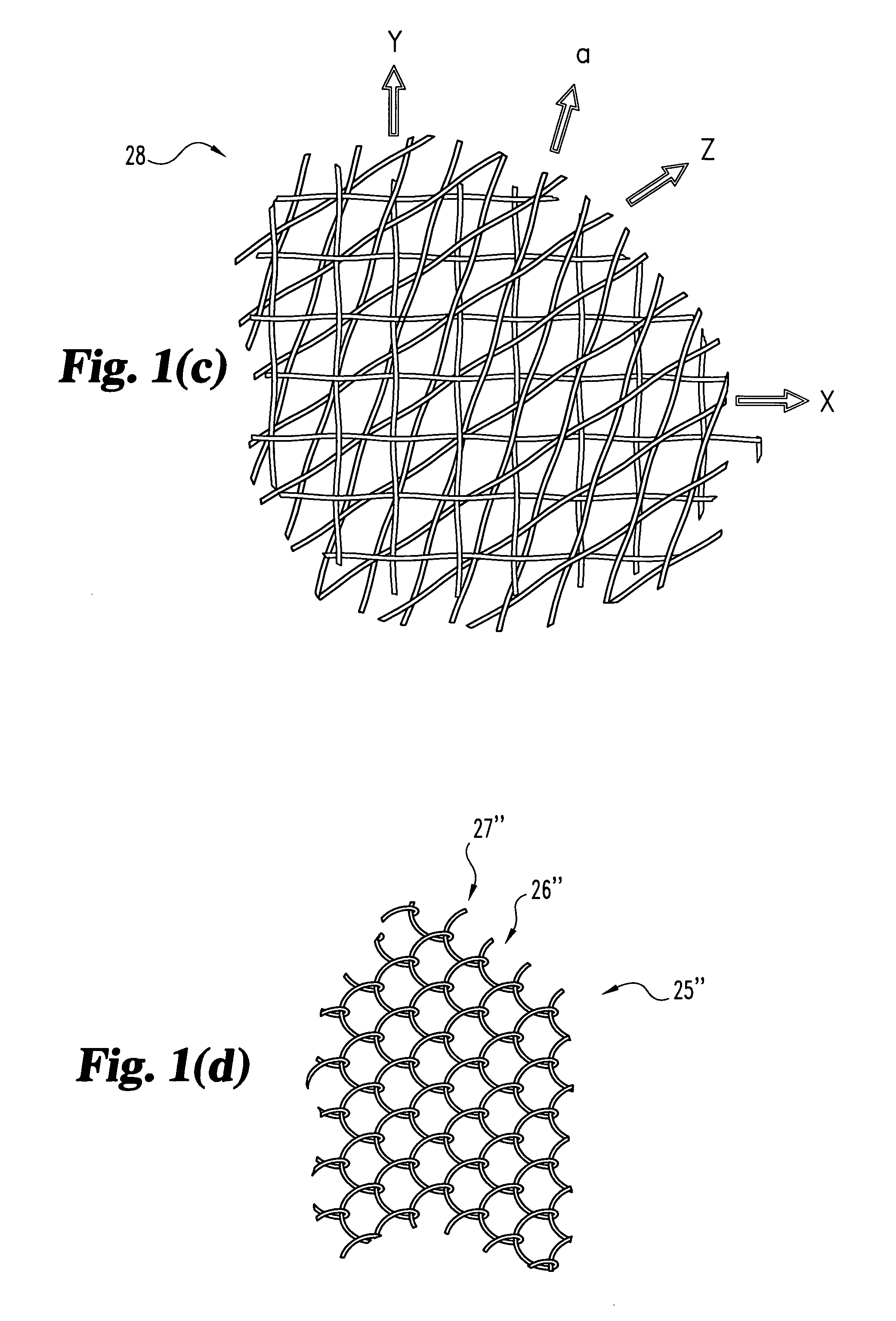
Woven orthopedic implants
InactiveUS20050043733A1Easy surgical implantationModulating bulk mechanical propertySuture equipmentsInternal osteosythesisTherapeutic implantProsthesis
The present invention relates to orthopedic implants made from a mesh material. The mesh material can be treated in order to promote bone growth, to provide antibiotics, or to provide other beneficial treatment. Specific applications for the implants include, for example, a prosthetic ligament, a tension band, an interbody device, or a fixation device that extends across one or more joints or fractures.
Owner:WARSAW ORTHOPEDIC INC
Woven orthopedic implants
InactiveUS7341601B2Easy surgical implantationModulating bulk mechanical propertyInternal osteosythesisBone implantProsthesisProsthetic ligament
The present invention relates to orthopedic implants made from a mesh material. The mesh material can be treated in order to promote bone growth, to provide antibiotics, or to provide other beneficial treatment. Specific applications for the implants include, for example, a prosthetic ligament, a tension band, an interbody device, or a fixation device that extends across one or more joints or fractures.
Owner:WARSAW ORTHOPEDIC INC
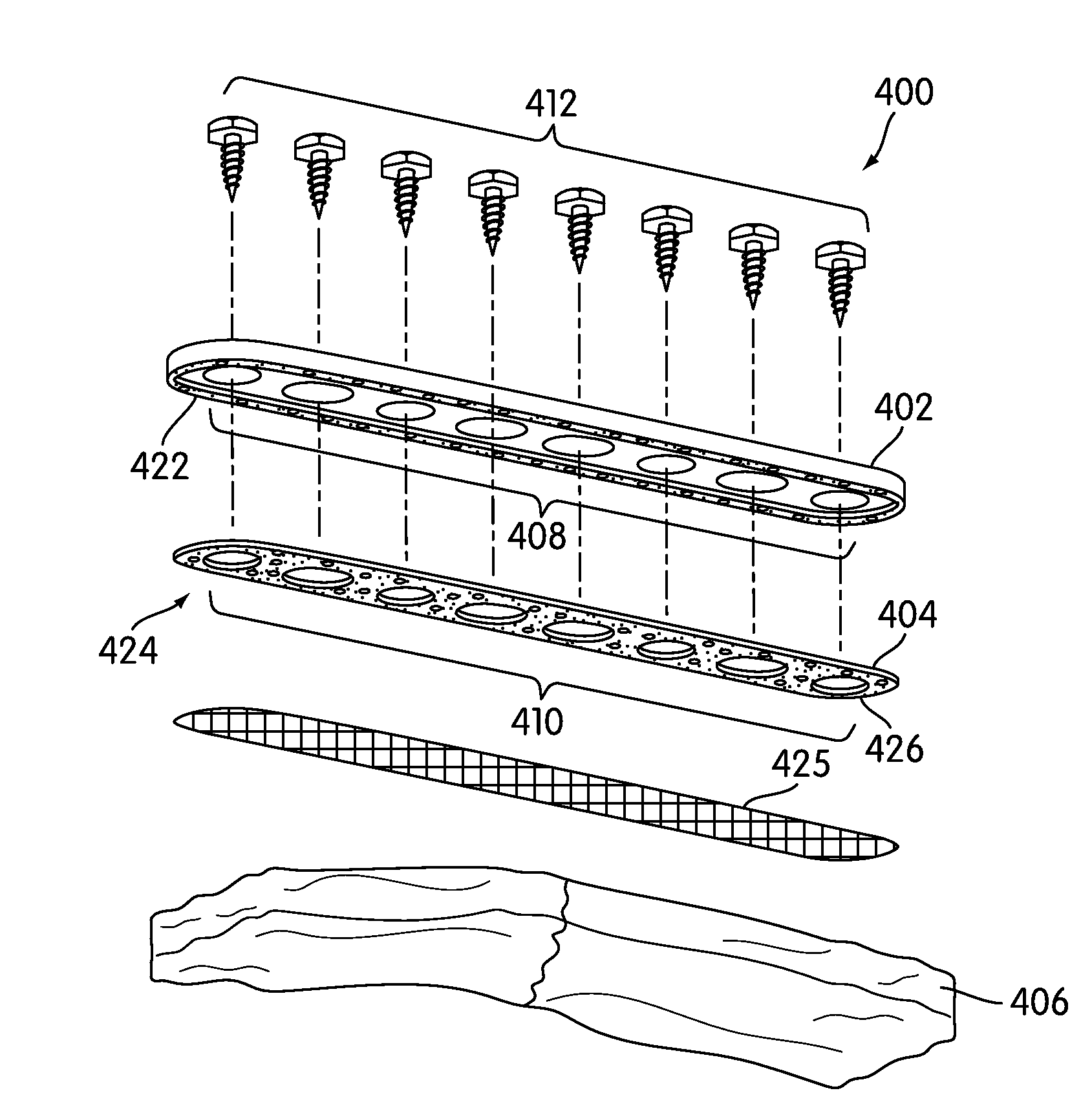
Spinal fusion apparatus and method
InactiveUS6926737B2Relieve stressPhysical recoveryInternal osteosythesisBone implantCushioningVertebral bone
An apparatus for stabilizing and promoting fusion between adjacent vertebrae includes at least a pair of implants to promote bone growth and to fuse with vertebral bone. The implants are joined by a connector. Preferably the implants are inserted into receiving bores in a non-parallel configuration and / or the connector joins the implants so as to bias the implants to a non-parallel configuration. A pair of connecting members also preferably secure the implants to each of the adjacent vertebrae. A method of using the apparatus provides for stabilizing between vertebrae where the original cushioning disc has deteriorated or become damaged. The implants are connected together. Also in the method, the implant receiving bores are non-parallel and / or the implants are biased to non-parallel configurations by joining the implants to the connecting element so as to reduce the inadvertent disturbance of the implants from the receiving bores and to further stabilize the implants overall during the fusion process.
Owner:WARSAW ORTHOPEDIC INC
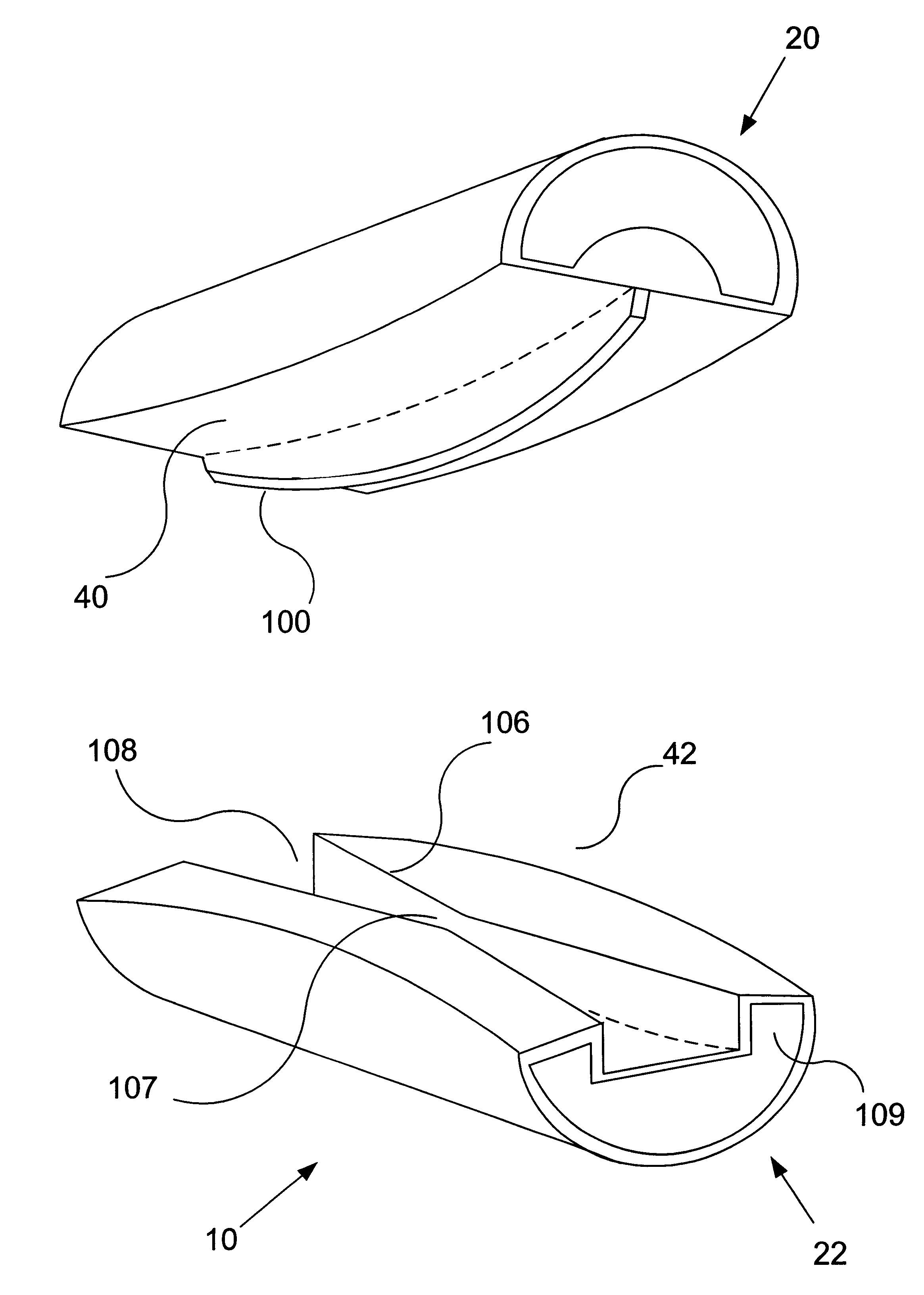
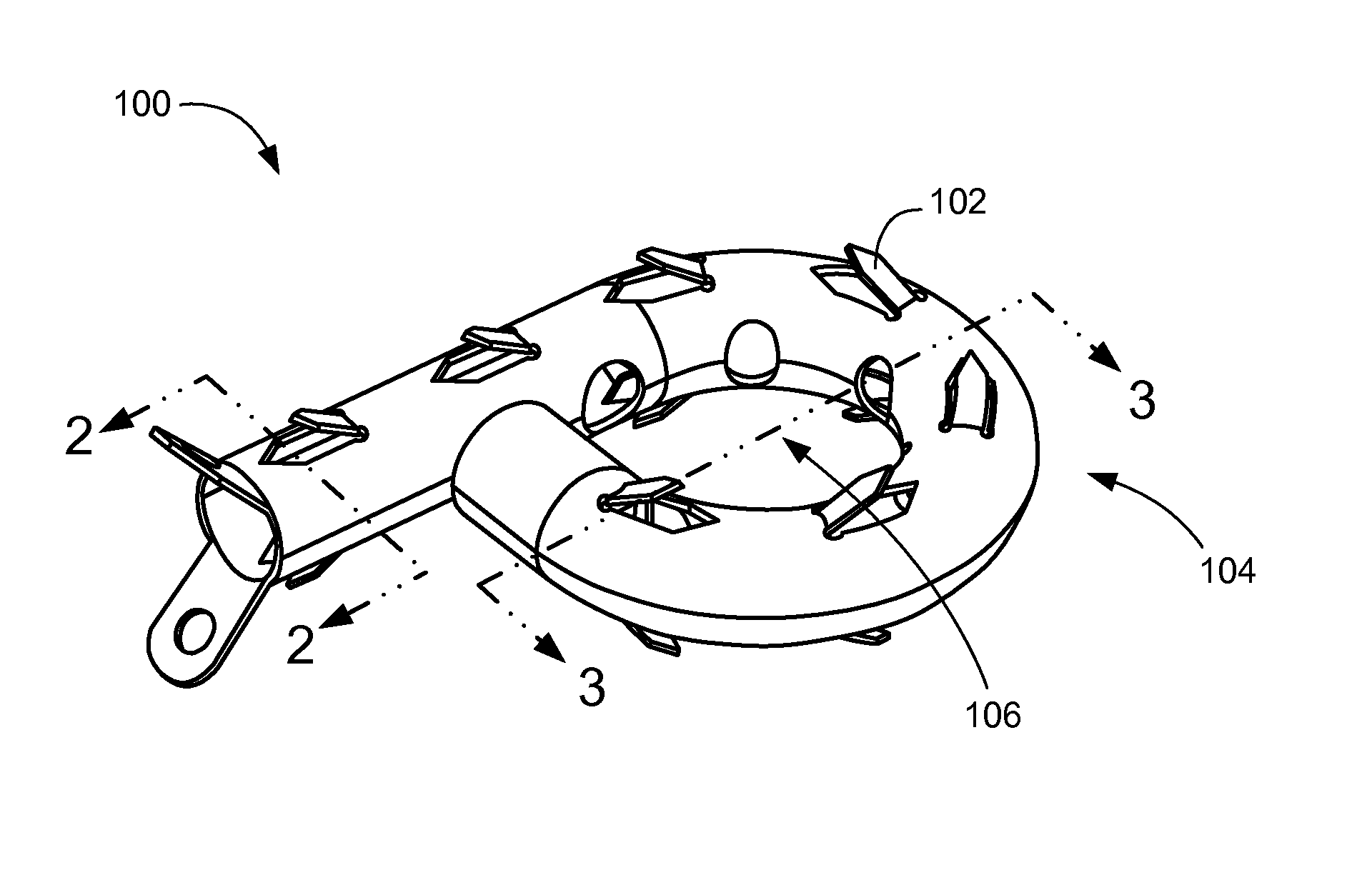
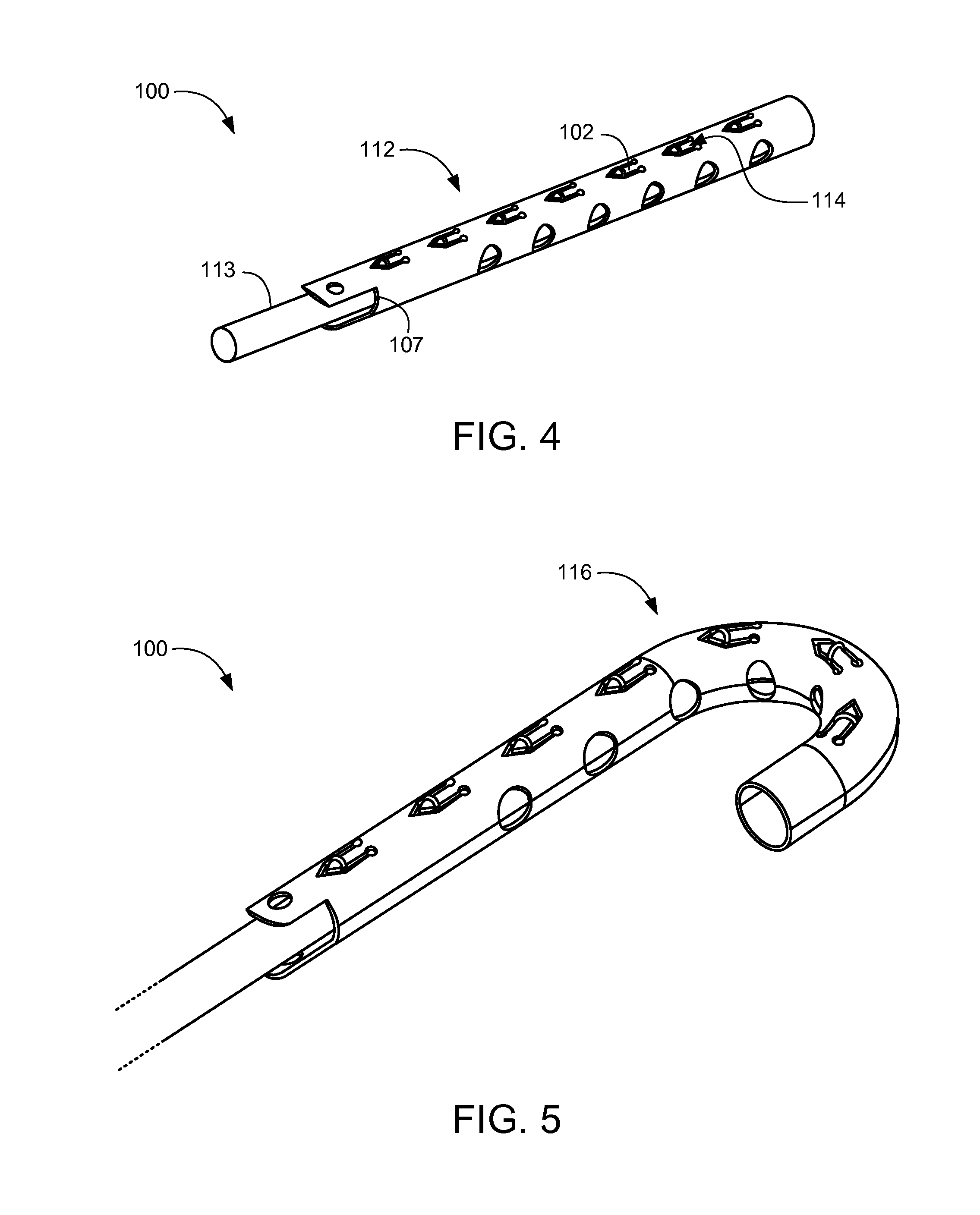
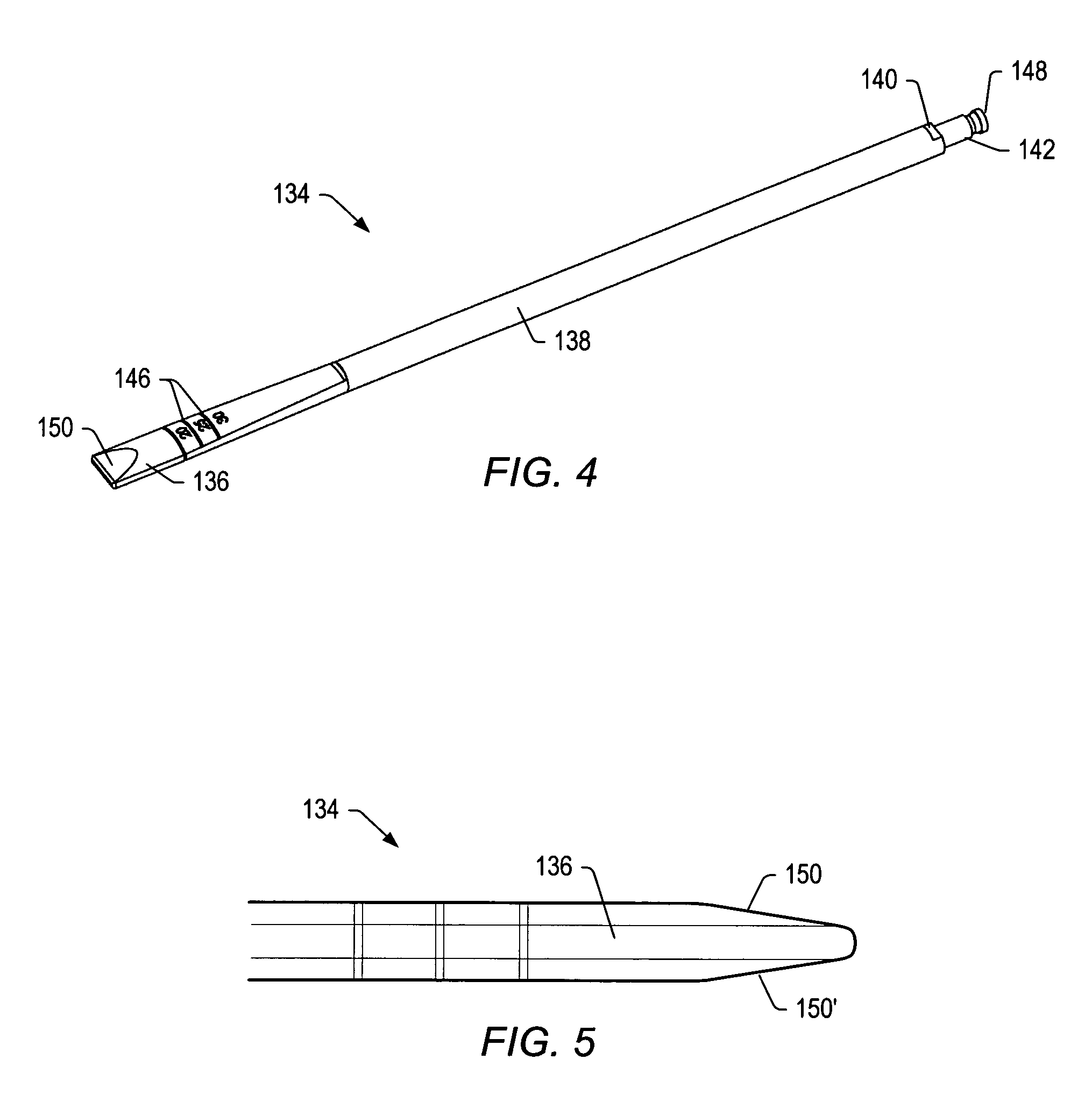
Axial spinal implant and method and apparatus for implanting an axial spinal implant within the vertebrae of the spine
Spinal implants for fusing and / or stabilizing spinal vertebrae and methods and apparatus for implanting one or more of such spinal implants axially within one or more axial bore within vertebral bodies in alignment with a visualized, trans-sacral axial instrumentation / fusion (TASIF) line in a minimally invasive, low trauma, manner are disclosed. Attachment mechanisms are provided that attach or affix or force the preformed spinal implants or rods to or against the vertebral bone along the full length of a TASIF axial bore or bores or pilot holes or at the cephalad end and / or caudal end of the TASIF axial bore or bores or pilot holes. The engagement of the vertebral body is either an active engagement upon implantation of the spinal implant into the TASIF axial bore or a passive engagement of the external surface configuration with the vertebral bone caused by bone growth about the external surface configuration. A plurality of such spinal implants can be inserted axially in the same TASIF axial bore or pilot hole or separately in a plurality of TASIF axial bores or pilot holes that extend axially and in a side-by-side relation through the vertebrae and discs, if present, between the vertebrae. Discectomies and / or vertebroblasty can be performed through the TASIF axial bore or bores or pilot holes prior to insertion of the spinal implants. Vertebroblasty is a procedure for augmentation of collapsed vertebral bodies by pumped-in materials, e.g., bone cement or bone growth materials. Materials or devices can also be delivered into the disc space to separate the adjoining vertebrae and / or into damaged vertebral bodies or to strengthen them.
Owner:TRANSI
Surgical implants for use as spinal spacers
A spinal implant is provided which maintains intervertebral spacing and stability within the spine. In some embodiments, two or more spinal implants may interlock to form a spinal stabilization system. Spinal implants may interlock using protrusions, indentations, teeth, and / or grooves. In an embodiment, an opening may be positioned in the spinal implant to fuse the spinal implant to surrounding bone tissue. Bone growth through the opening may be increased by using a removable bone growth stimulating insert in the opening. A spinal implant may be constructed of biocompatible material, for example, bone, metal, and / or polymers.
Owner:SPINAL CONCEPTS
Malleable paste for filling bone defects
InactiveUSRE38522E1Easy to packFast absorptionSurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
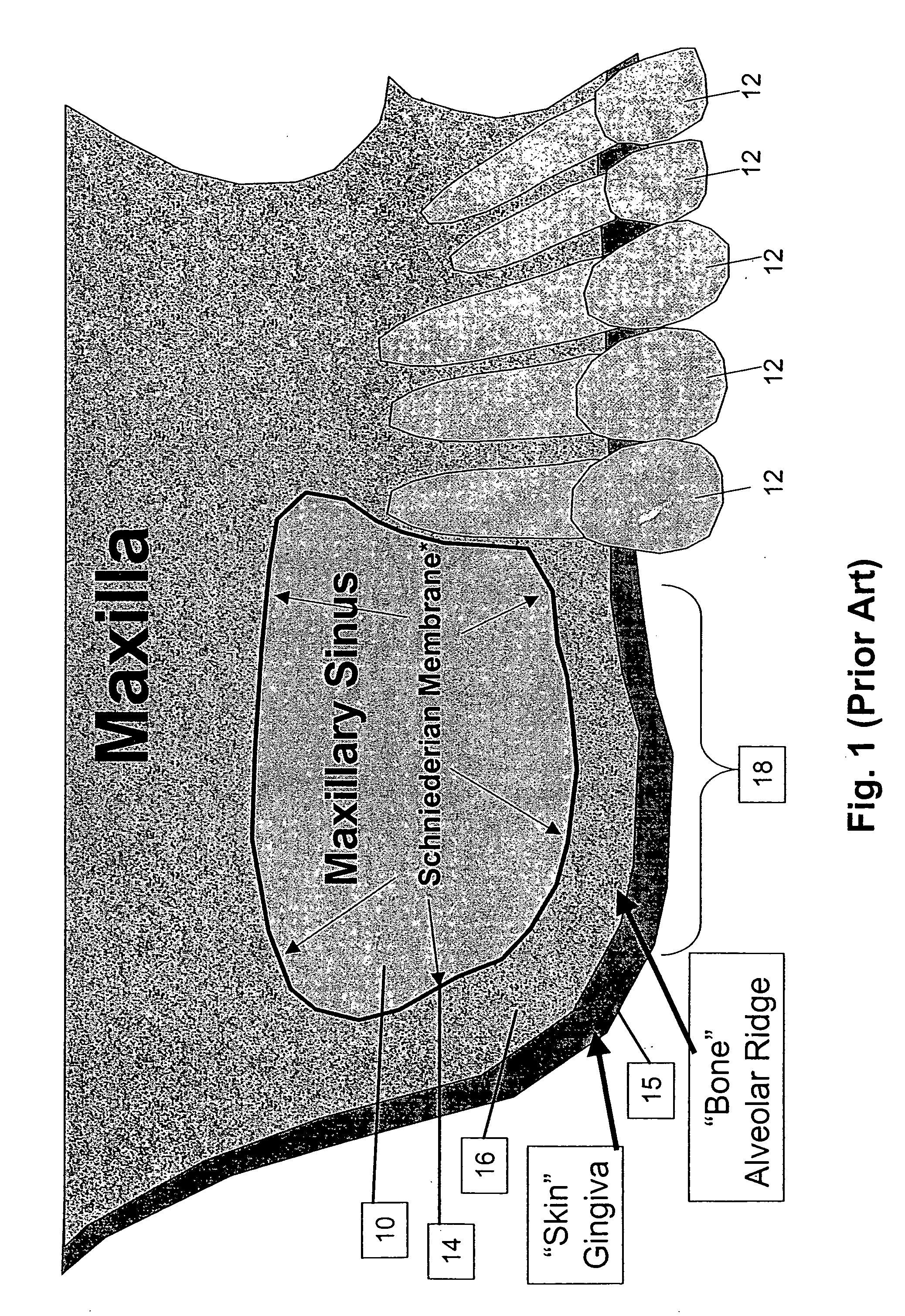
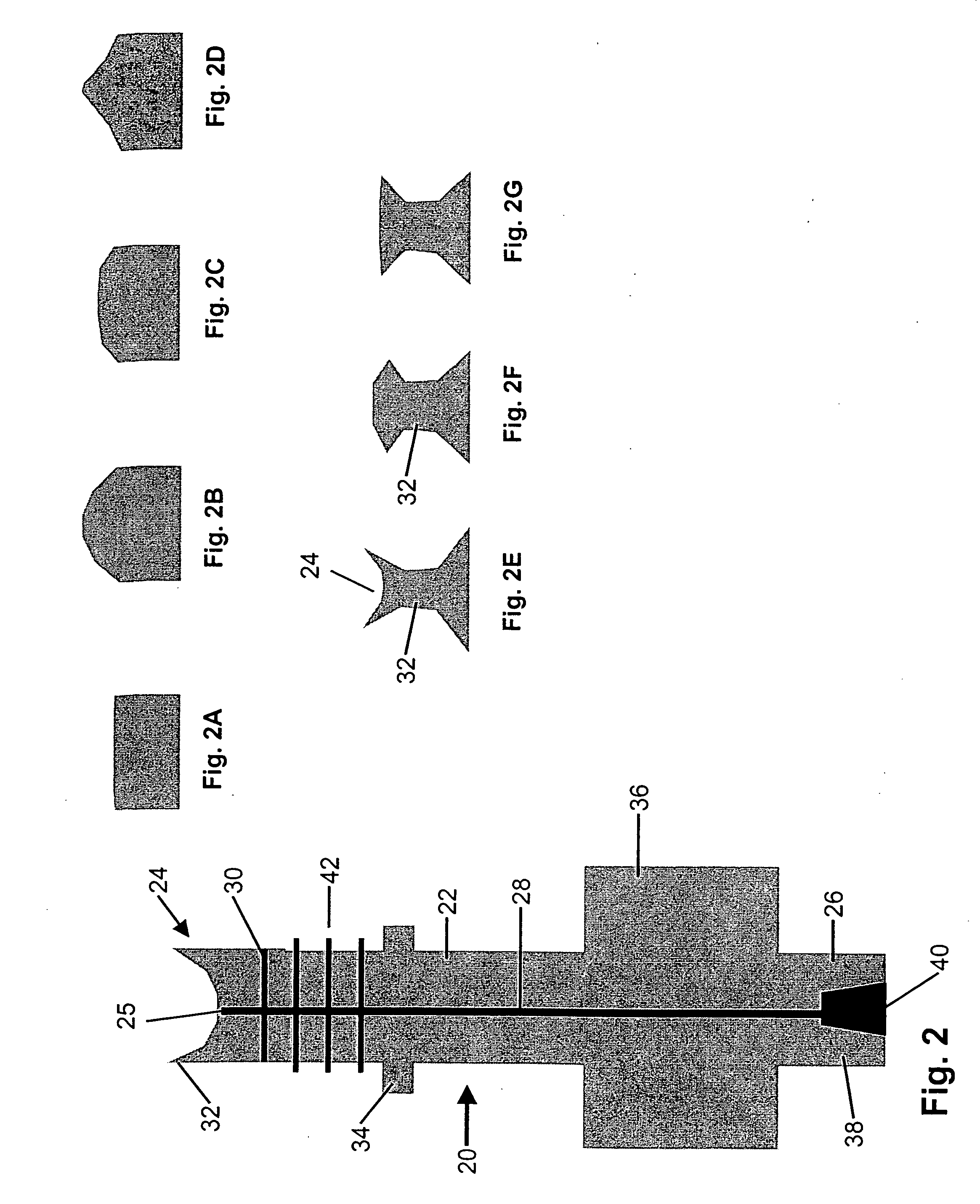
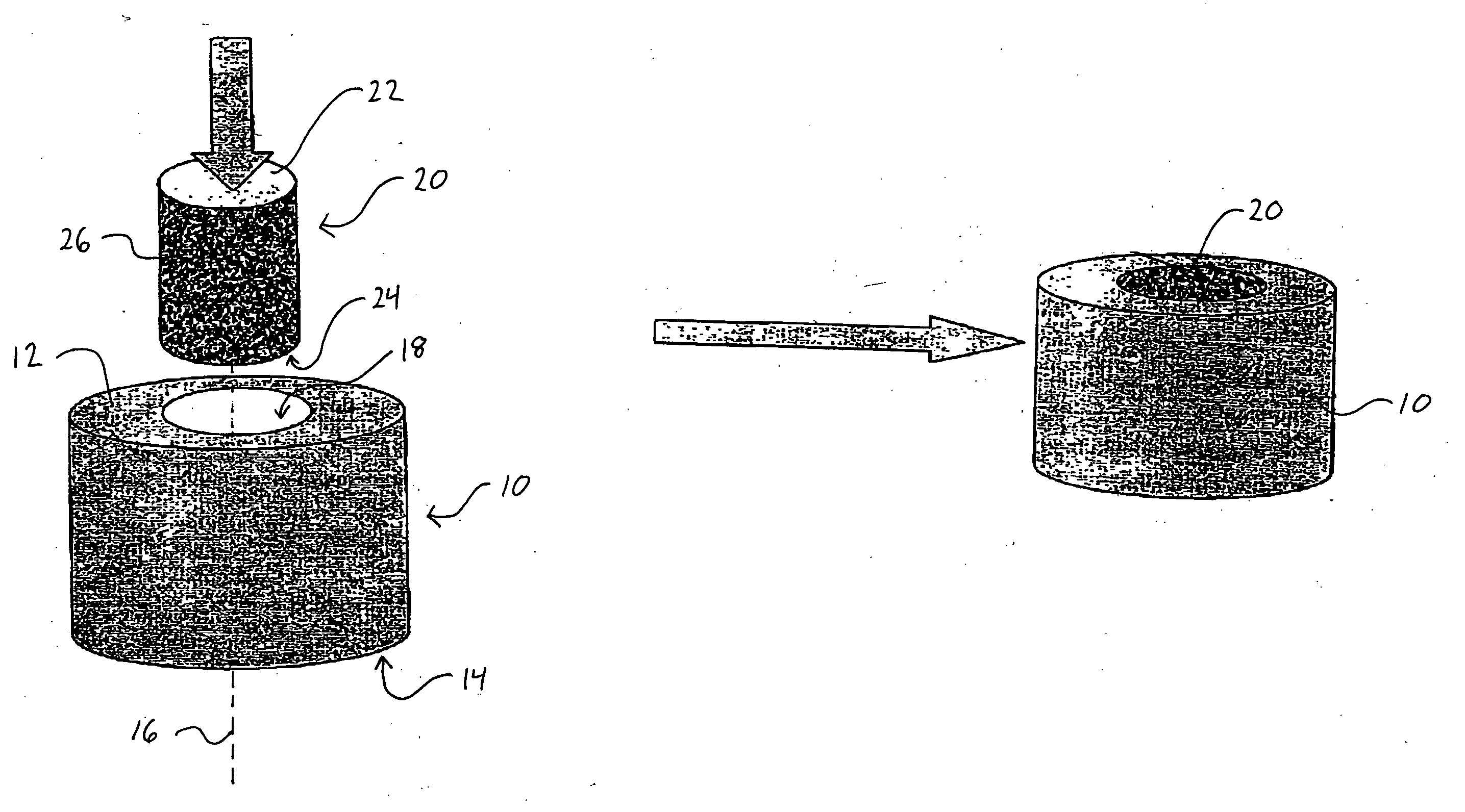
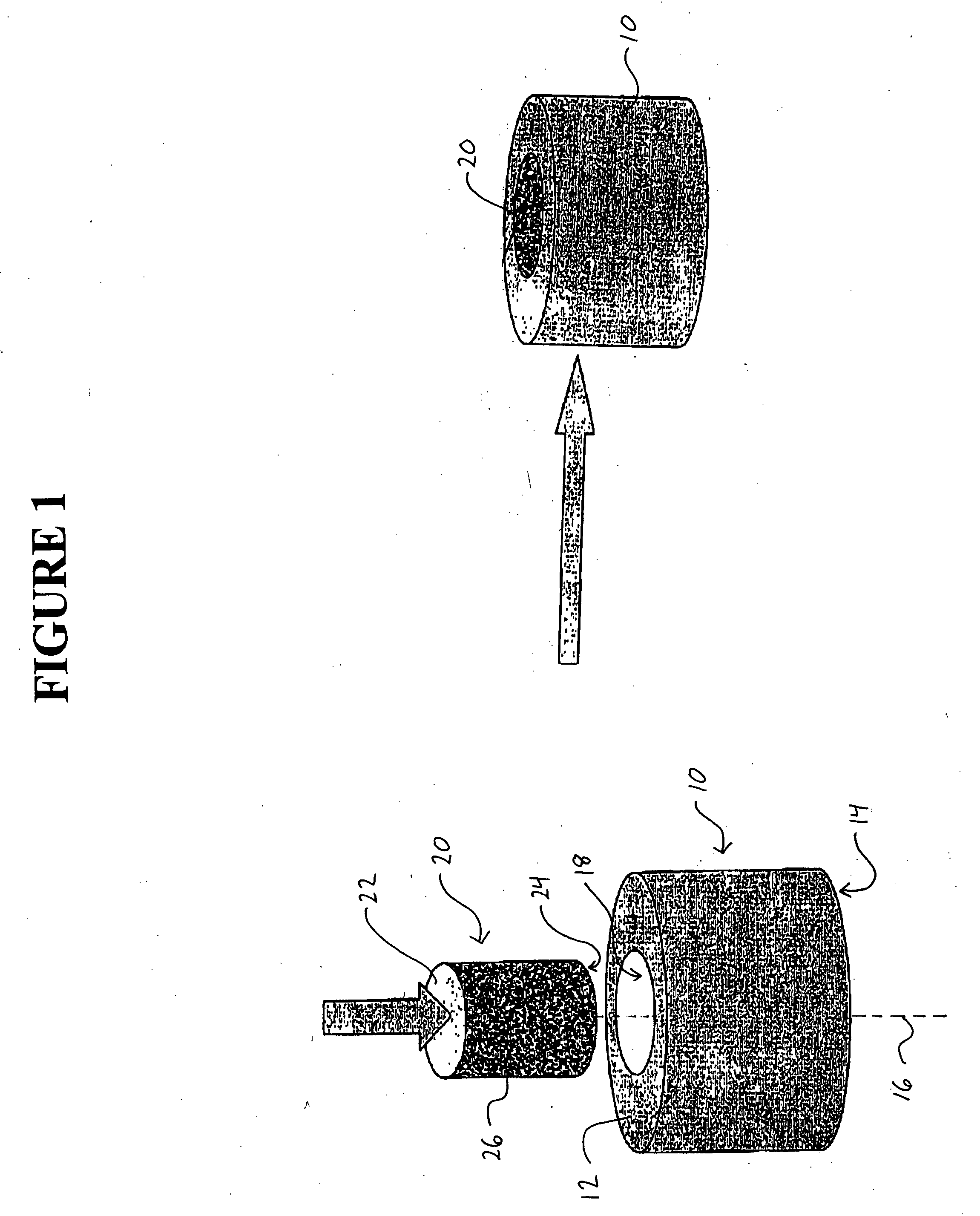
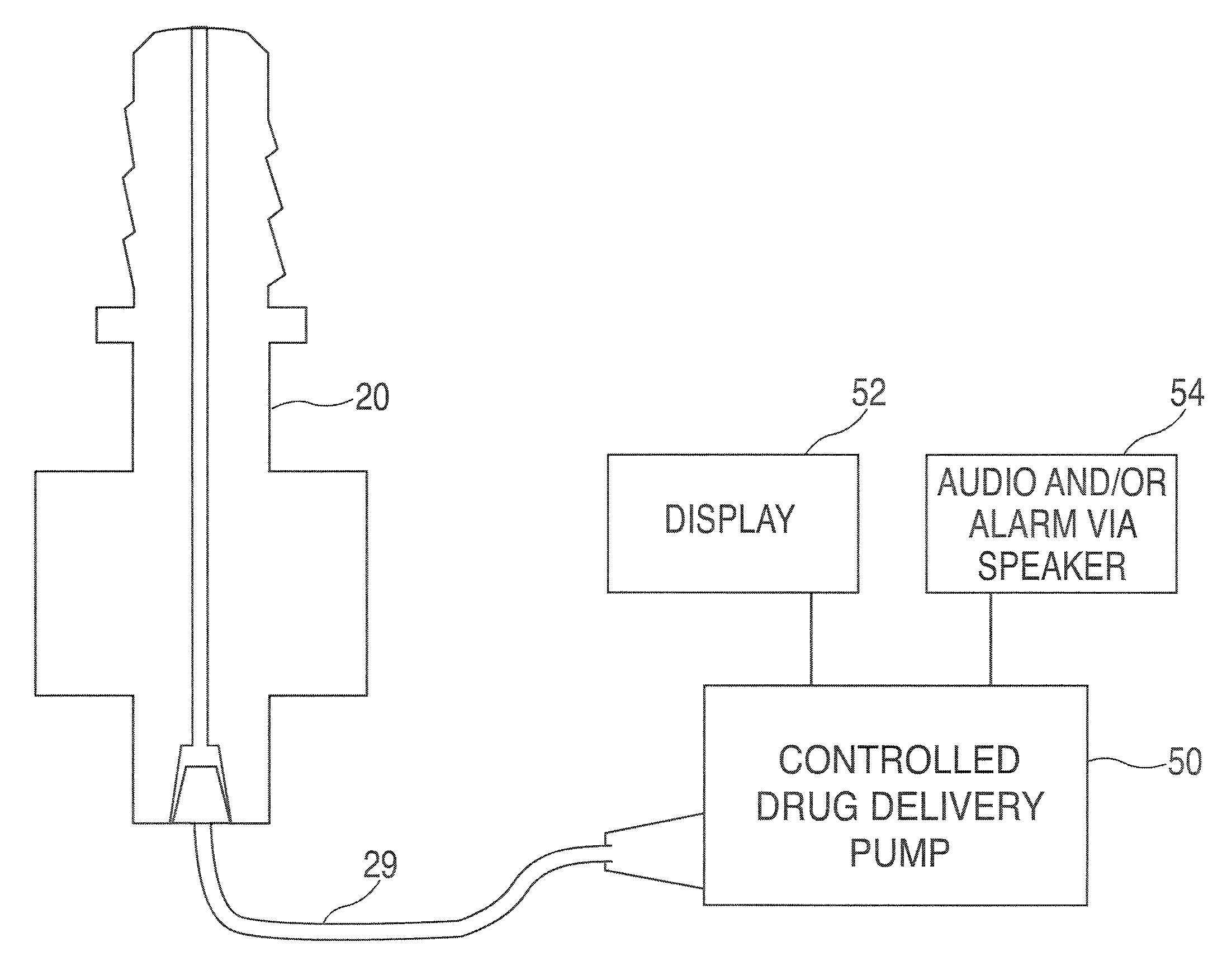
Method and apparatus for performing maxillary sinus elevation
InactiveUS20060084034A1Miss massEffective placementDental implantsFastening prosthesisBone growth stimulatorBiomedical engineering
A method and apparatus is disclosed for providing implants in the upper jaws of a person. A sleeve is inserted through the alveolar ridge to the maxillary sinus. The sleeve is used to raise the subantral membrane and form a cavity. A filler, such as a bone growth stimulant is injected through the sleeve into the cavity. In the process, the sleeve also can cut and / or condense the bone around itself so that the bone can hold an implant. Optionally, the bone growth stimulant is also introduced into the bone surrounding the sleeve. During the injection, the pressure within the sleeve or the cavity is monitored to detect and prevent the rupture of the subantral membrane.
Owner:HOCHMAN MARK N
Osteoconductive integrated spinal cage and method of making same
The spinal cage comprises a structural component having sufficient strength to withstand the compressive loading between vertebral bodies. The structural component is integrated with an osteoconductive component to facilitate bone growth between the vertebral bodies. The structural component may comprise any of PEEK, PEKK, or other structural material. The osteoconductive component may comprise any of allograft, natural bone, tricalcium phosphate, hydroxyapatite or a blend of calcium carbonate, calcium lactate and other calcium salts. A method for making the spinal cage involves molding polymers around an osteoconductive component, heat staking, and may further include ultrasonically welding, snap fit or mechanically assembling and / or adhesively bonding components.
Owner:SPINAL ELEMENTS INC
Memory material implant system and methods of use
InactiveUS20140067073A1Minimize possible migrationImprove structural rigiditySuture equipmentsInternal osteosythesisDistractionImplanted device
Apparatus and method used to reduce the movement between vertebrae or fractured bones. The implantable device can be deformed from its shape-set configuration for ease of deployment and return to a pre-set shape upon completion of deployment. The apparatus can serve to stabilize fractured bones or as a distraction device and support structure between vertebrae. Device may be made of a material with shape memory and superelastic properties which facilitate the method of implantation. The pre-set shape of the device may include dimensions / geometries which are similar to the natural curvature of the human spine or bone without the use of hinging or connection between multiple pieces. Once deployed, the device can serve to constrain the flow of bone growth material between the inside and outside of the device.
Owner:HAUCK BRIAN ALBERT
Osseo-integrated sub-periosteal implant
A sub-periosteally implantable prosthesis support structure for a fixed or detachable dental prosthesis includes a framework fitted to and generally conforming to the inner and outer contours of the bony ridge structures of a person. The framework is configured to provide a space extending generally normal to the bony ridge structure to an apex to provide space for subsequent bone growth. A plurality of denture support posts are distributed about the framework and depend outwardly from the apex in substantial alignment with the bony ridge structure. During the fabrication of the prosthesis support structure, a bio-compatible fine mesh screen is fixed to and spans, tent-like, the framework to substantially overlay the bone structure and the space provided for subsequent bone growth. After the support structure has been implanted, the growth of bone into the space and around the support structure is promoted to osseo-integrate the support structure with the person's bony ridge, thus providing a secure foundation for a denture or fixed dental prosthesis configured for detachable or fixed coupling with the denture support posts. The support structure may be made, partly or wholly, from either non-resorbable material, such as titanium stock and mesh, or from a resorbable material such as Vicryl TM .
Owner:ROBINSON DANE Q
Method of inducing new bone growth in porous bone sites
InactiveUS6599520B2High densityReduce porosityInternal osteosythesisSurgical adhesivesPorosityVolumetric Mass Density
A method of treating a condition in a vertebrate animal characterized by bone having increased porosity and / or decreased bone mineral density. The porous bone is injected with an effective amount of a flowable bone composition. Also provided is a kit for the treatment of porous bone wherein a flowable bone composition is contained within an injectable delivery system.
Owner:WARSAW ORTHOPEDIC INC
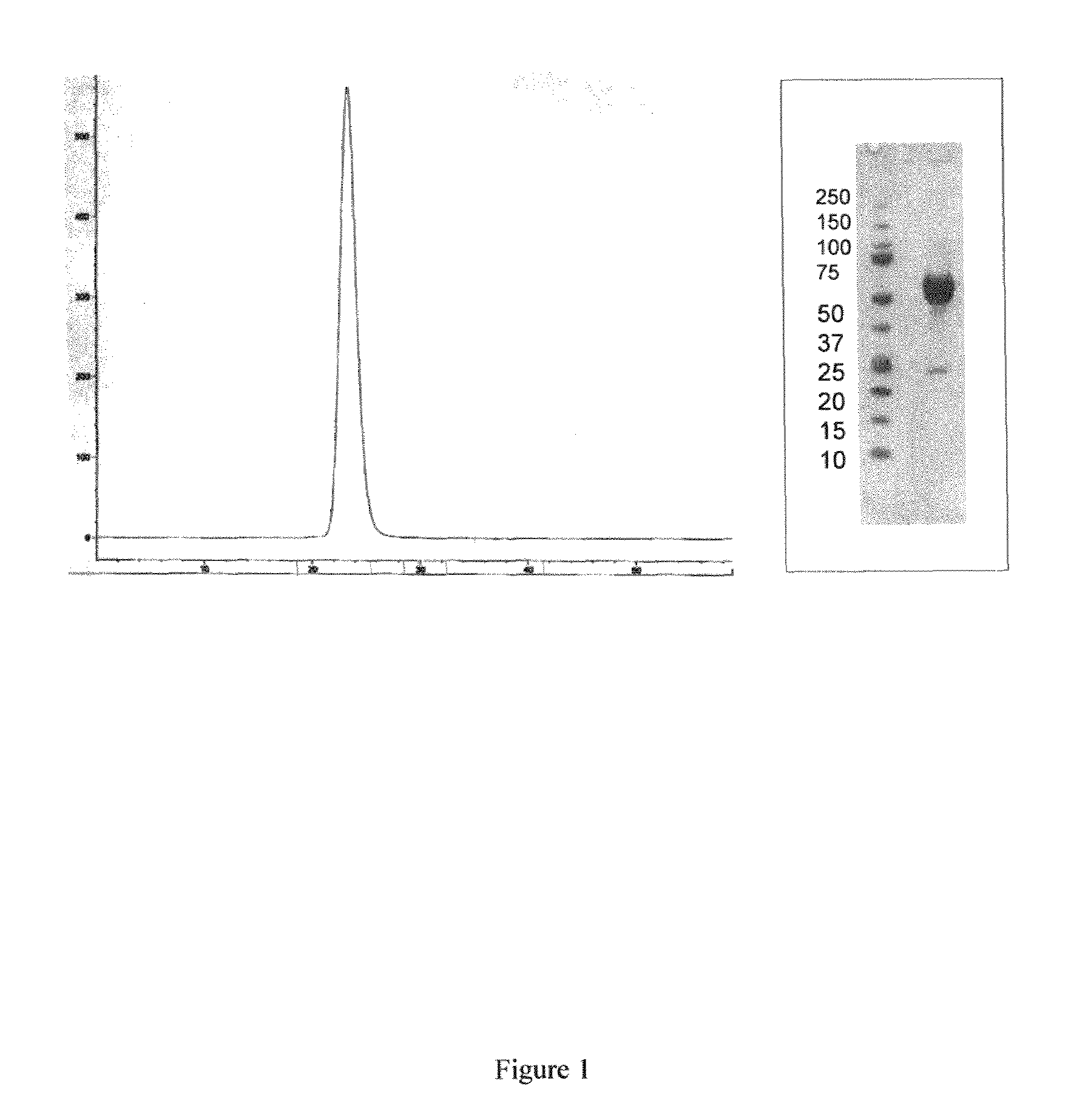
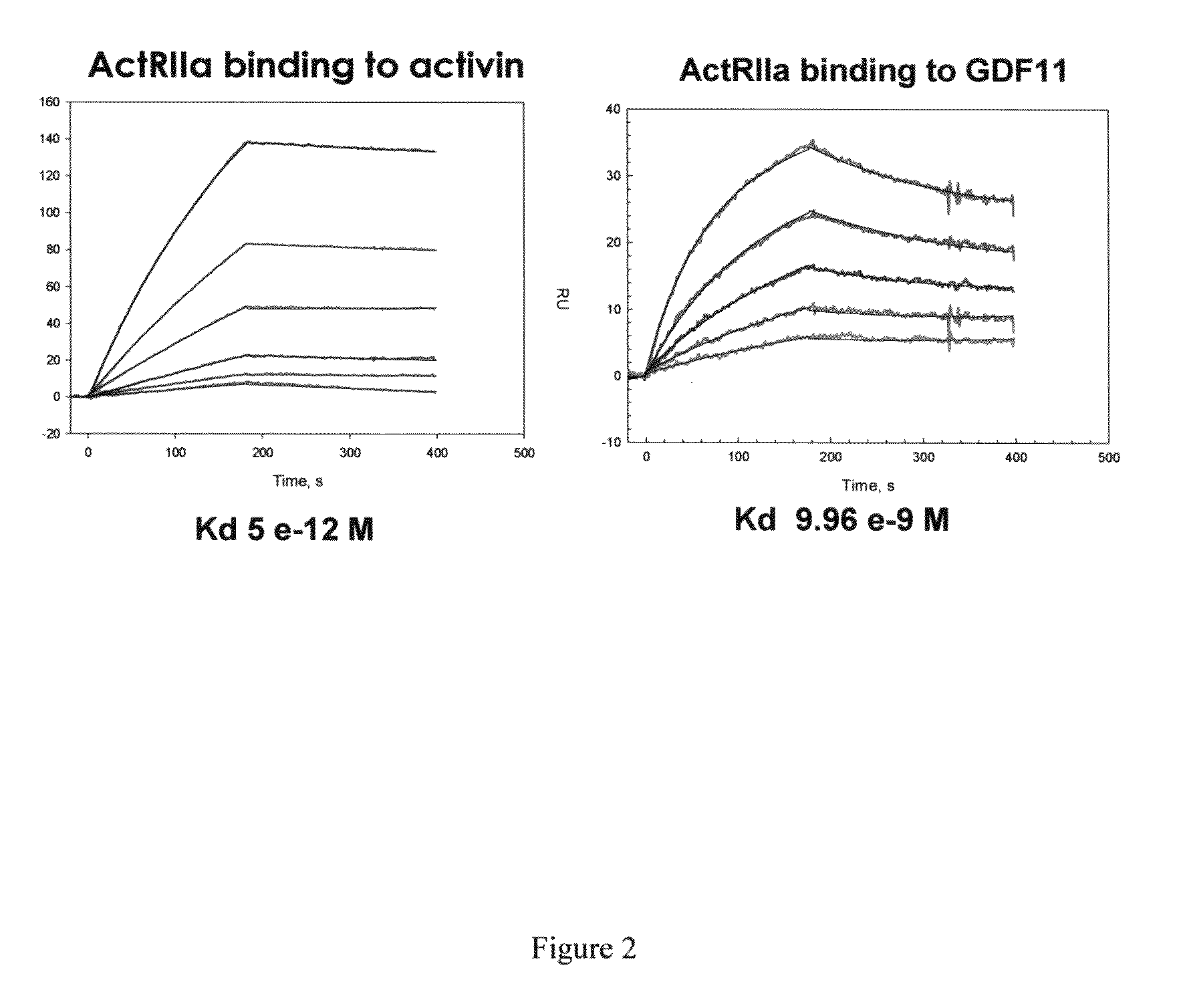
Activin-ActRIIa antagonists and uses for promoting bone growth
ActiveUS20090099086A1Reduce expressionPromote bone growthPeptide/protein ingredientsAntibody mimetics/scaffoldsIncreased Bone DensityCancer research
In certain aspects, the present invention provides compositions and methods for promoting bone growth and increasing bone density.
Owner:ACCELERON PHARMA INC
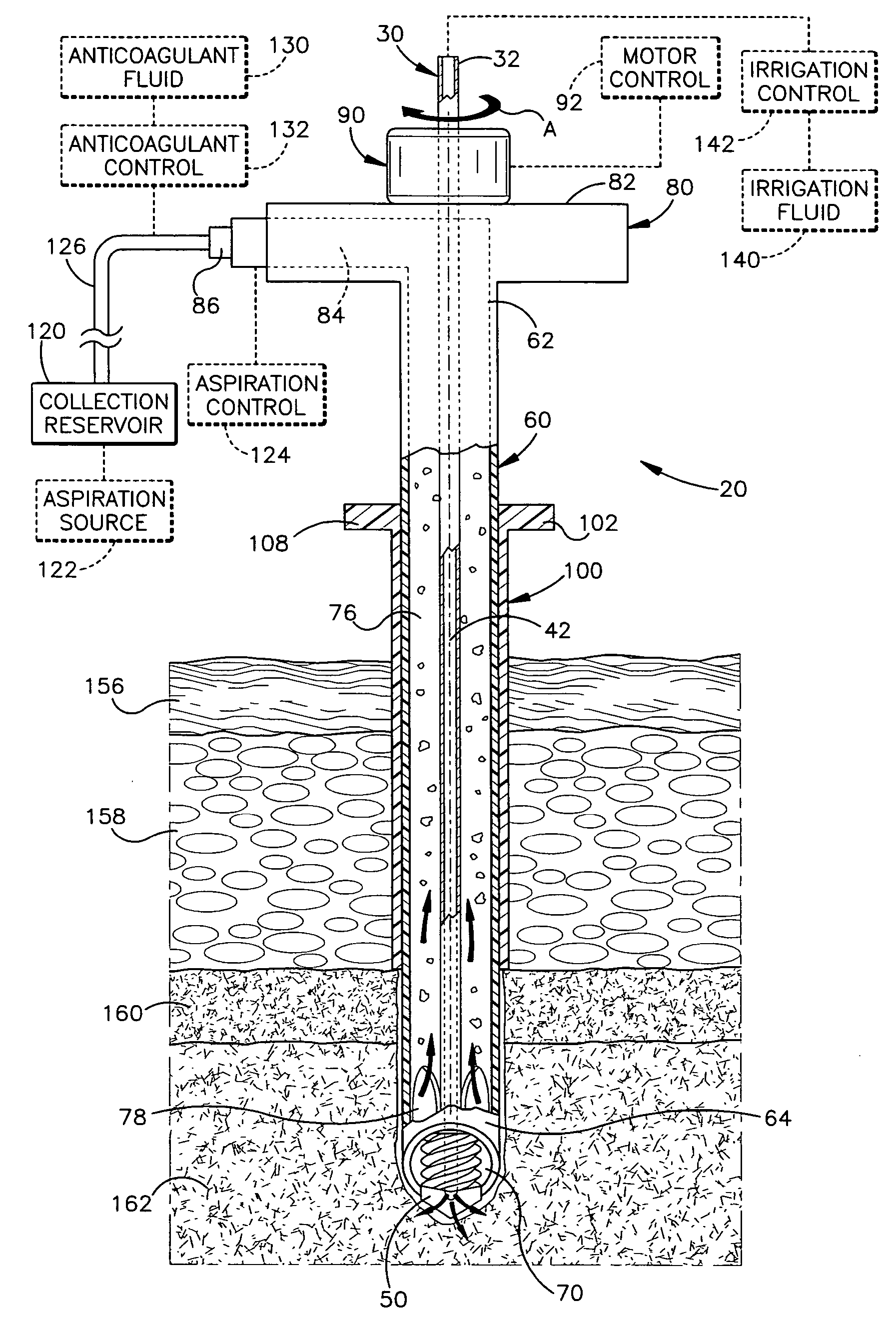
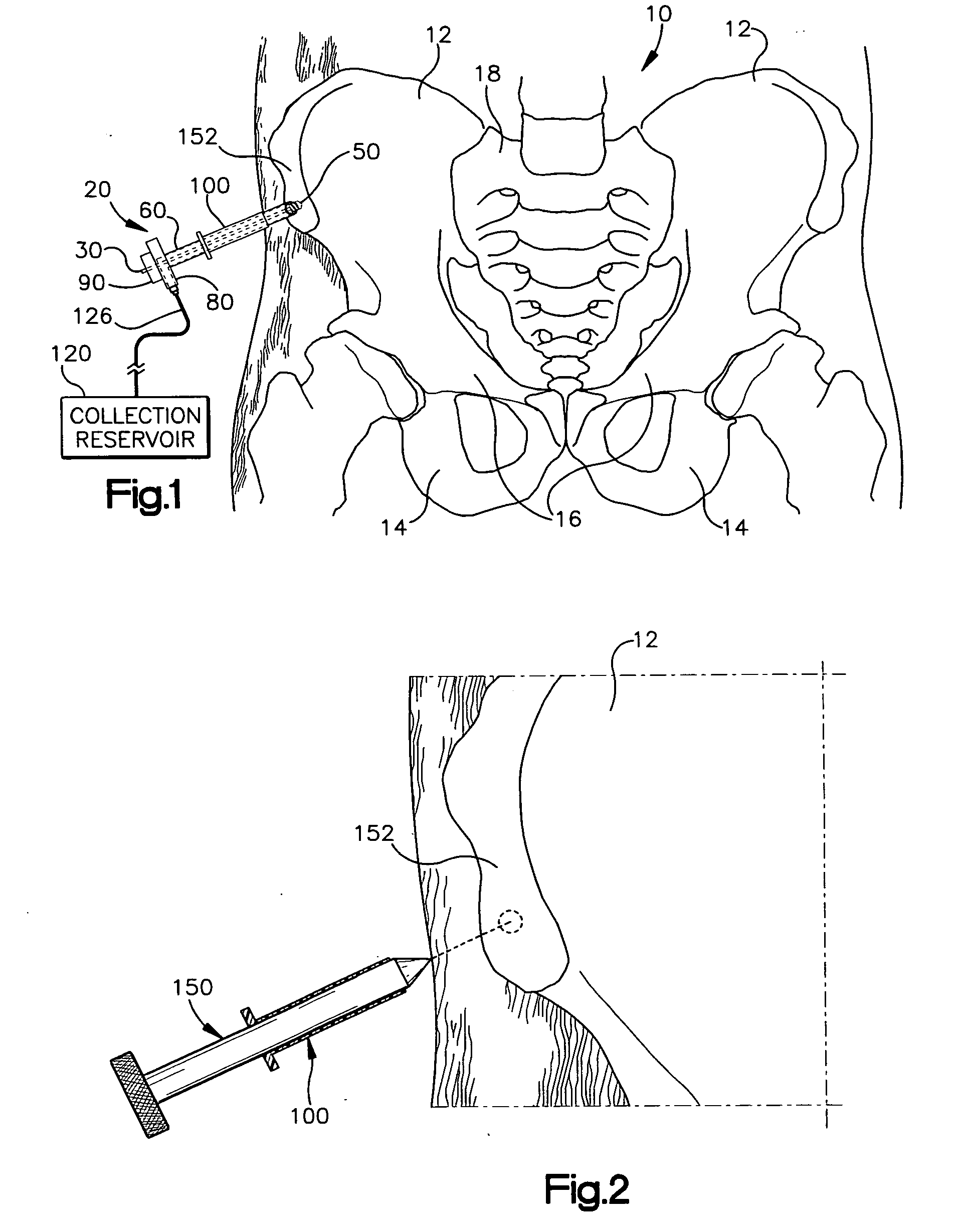
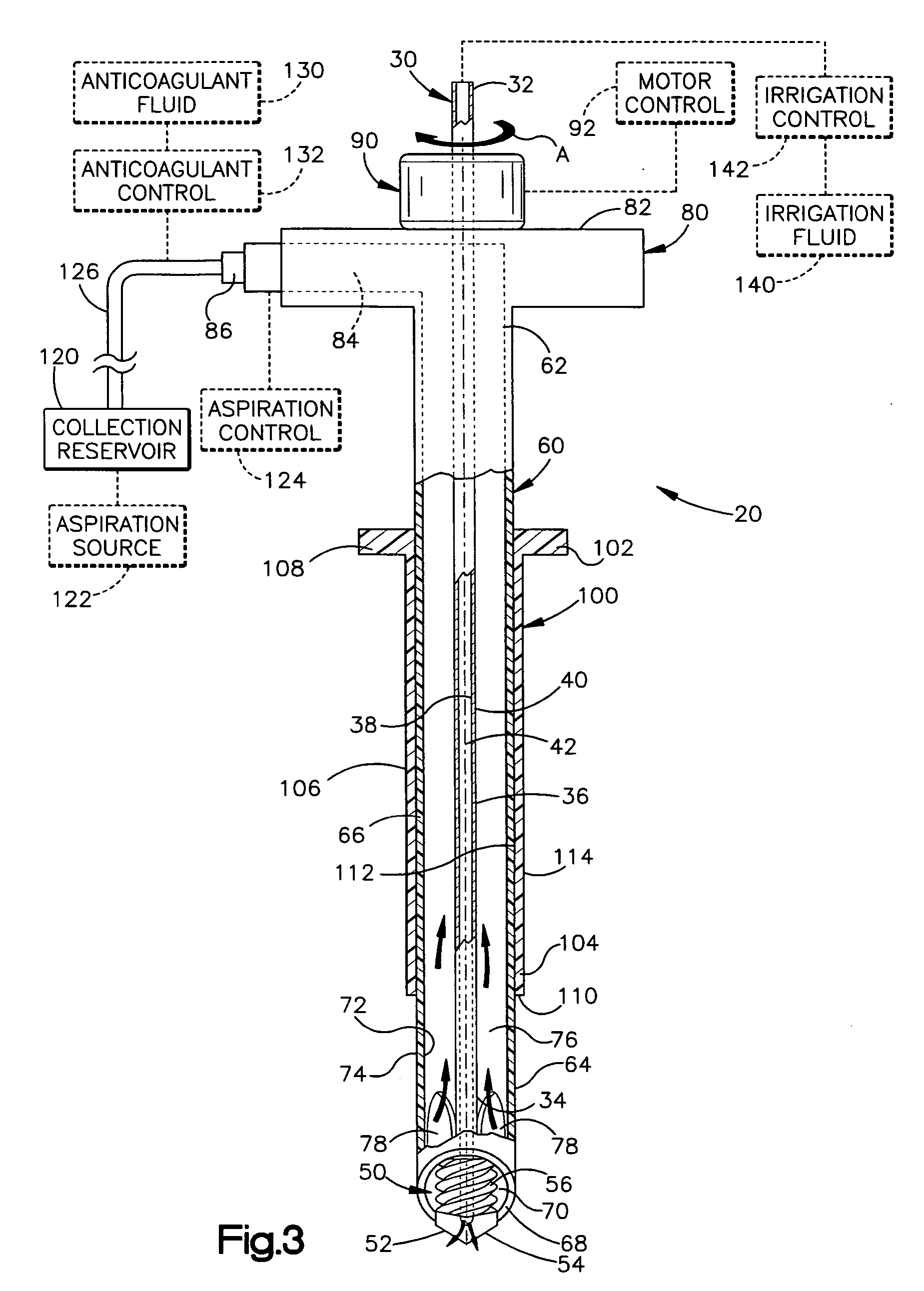
Apparatus and method for harvesting bone marrow
InactiveUS20070055282A1Minimally invasivePromote bone healingSurgical needlesVaccination/ovulation diagnosticsBone tissueBone splinters
A minimally invasive apparatus and method for harvesting bone marrow cells, blood, and bone fragments includes a rigid cannula having a proximal end and a distal end with an opening. The distal end includes a cutting tip that is movable axially and radially to cut and disrupt bone tissue while preserving necessary viability among harvested marrow cells. The cannula further includes an inner surface defining an internal passage that extends from the opening toward the proximal end. Suction is applied to the passage to draw disrupted bone marrow cells, blood, and bone fragments into the internal passage for collection. The apparatus may include a rotatable shaft disposed co-axially within the internal passage. The shaft has a distal end with a cutting bit for further cutting and disrupting of bone tissue, and a lumen for supplying bone cement to the cutting bit to promote bone growth and healing within the bone.
Owner:THE CLEVELAND CLINIC FOUND
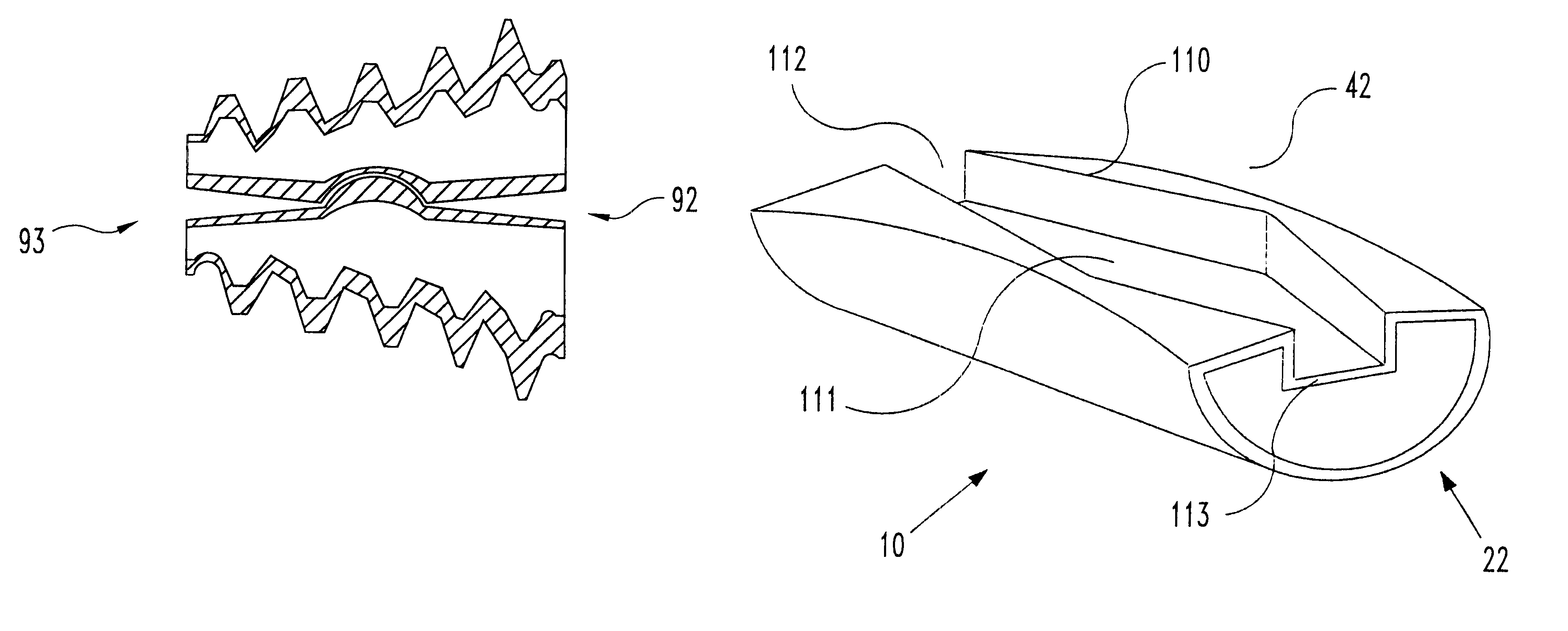
Spinal implant
Owner:ZIMMER BIOMET SPINE INC
Spinal Fusion Implants with Selectively Applied Bone Growth Promoting Agent
A spinal fusion device including a selectively applied bone growth promoting agent is disclosed. In particular, a bone growth promoting agent is selectively applied to spinal implants, spinal plugs, spinal wedges and other implantable devices.
Owner:JMEA CORP
Bone implants and methods
The disclosure provides implants, instruments and methods for bone fusion procedures. In some embodiments, the implants are particularly advantageous for use between opposing vertebral bodies to facilitate stabilization or arthrodesis of an intervertebral joint. The implants include, at least, a support component that provides structural support during fusion. In a typical embodiment, the implants also include a growth component. A growth component provides an environment conducive to new bone growth between the bones being fused. Several unique configurations to enhance fusion, instruments for insertion and methods for insertion are also disclosed.
Owner:ZIMMER SPINE INC
Method and apparatus for performing maxillary sinus elevation
InactiveUS7510397B2Miss massEffective placementDental implantsFastening prosthesisPeritoneumBiomedical engineering
A method and apparatus is disclosed for providing implants in the upper jaws of a person. A sleeve is inserted through the alveolar ridge to the maxillary sinus. The sleeve is used to raise the subantral membrane and form a cavity. A filler, such as a bone growth stimulant is injected through the sleeve into the cavity. In the process, the sleeve also can cut and / or condense the bone around itself so that the bone can hold an implant. Optionally, the bone growth stimulant is also introduced into the bone surrounding the sleeve. During the injection, the pressure within the sleeve or the cavity is monitored to detect and prevent the rupture of the subantral membrane.
Owner:HOCHMAN MARK N
Dental implants and methods for their fabrication and use
InactiveUS20050048440A1Enhance post-implant boneEnhance ligament growthDental implantsTeeth fillingAbutmentDental implant
A dental implant for use in replacing a nonfunctional tooth includes an abutment and a base. The base of the implant has a topography this is substantially identical to the topography of the root of the nonfunctional tooth. Accordingly, the use of the implants eliminates the need for conventionally used bone drills and other traumatic preparing procedures for implant. The implant may be fabricated from a single piece of material so that the abutment and the base are unitary. In addition, the surface of the base may be treated to enhance post-implant bone growth to the base.
Owner:FENG JAMES C
Arthroplasty devices configured to reduce shear stress
InactiveUS20050143837A1Increase capacityImproved ingrowthInternal osteosythesisBone implantShear stressSacroiliac joint
Arthroplasty devices having improved bone in growth to provide a more secure connection within the body. Different embodiments disclosed include devices having threaded intramedullary components, devices configured to receive bone growth promoting substances, devices with resorbable components, and devices configured to reduce shear stress.
Owner:FERREE BRET A
Arthroplasty devices configured to reduce shear stress
InactiveUS20040019386A1Reduce shear stressPromote bone growthInternal osteosythesisDiagnosticsShear stressSacroiliac joint
Arthroplasty devices having improved bone in growth to provide a more secure connection within the body. Different embodiments disclosed include devices having threaded intramedullary components, devices configured to receive bone growth promoting substances, devices with resorbable components, and devices configured to reduce shear stress.
Owner:FERREE BRET A
Malleable paste for filling bone defects
InactiveUSRE39587E1Useful bulk viscosityAbsorb more quicklySurgical adhesivesPeptide/protein ingredientsBone defectBiomedical engineering
The invention is directed toward a malleable bone putty and a flowable gel composition for application to a bone defect site to promote new bone growth at the site which comprises a new bone growth inducing compound of demineralized lyophilized allograft bone powder. The bone powder has a particle size ranging from about 100 to about 850 microns and is mixed in a high molecular weight hydrogel carrier, the hydrogel component of the carrier ranging from about 0.3 to 3.0% of the composition and having a molecular weight of about at least 10,000 Daltons. The composition contains about 25% to about 40% bone powder and can be additionally provided with BMP's and a sodium phosphate buffer.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com