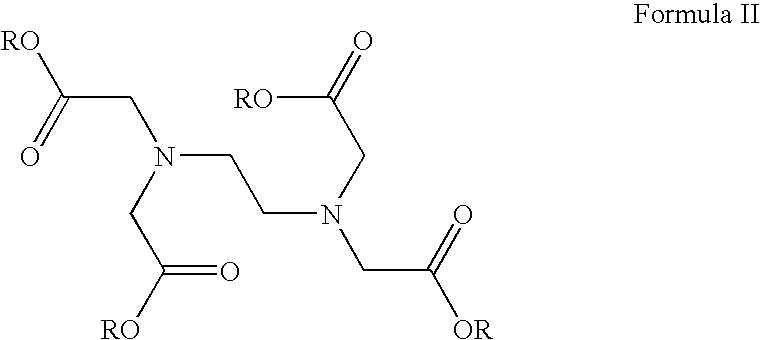
[0007]In certain embodiments, the present invention is directed to methods and pharmaceutical compositions for treating, preventing or alleviating the symptoms of and / or
inflammation associated with inflammatory diseases involving the
gastrointestinal tract, including the
esophagus,
stomach and / or
digestive tract. Provided herein are methods of treating, preventing or alleviating, for example, esophageal inflammation in an individual. In certain embodiments, these methods comprise orally administering to said individual a
corticosteroid in association with at least one additional
active agent. In some embodiments, provided herein is a pharmaceutical composition comprising a
corticosteroid and at least one additional
active agent. In specific embodiments, the at least one additional
active agent is an agent that treats, prevents, or alleviates the symptoms of and / or inflammation associated with inflammatory diseases involving the
gastrointestinal tract. In some embodiments, inflammatory diseases involving the gastrointestinal tract involve, by way of non-limiting example, the esophagus, the
stomach and / or the small intestines. In more specific embodiments, the at least one additional active agent is not a second
corticosteroid. In certain embodiments, the pharmaceutical composition further comprises a liquid vehicle. In further or alternative embodiments, the pharmaceutical composition is suitable for
oral administration. In some embodiments, the composition further comprises an
excipient or combination of excipients. In specific embodiments, the
excipient or combination of excipients increases the interaction of the composition and / or the corticosteroid and / or the at least one additional active agent with a surface of the gastrointestinal tract (e.g., the surface of the esophagus).
[0009]In certain embodiments, the present invention provides a new method for reducing the risk of gastrointestinal
reflux and esophageal inflammation in people taking corticosteroids for
pain relief and for other conditions, particularly during treatment. In some embodiments, the present invention provides a method for treating patients presenting with
GERD or
GERD-like symptoms, but may be afflicted also or instead with non-GERD
disease, such as
eosinophilic esophagitis (EE or EoE), in a safe and efficient manner. In some embodiments, the method involves the administration of a composition that combines: a) a corticosteroid; b) an
acid inhibitor (e.g., a H2
antagonist and / or a PPI) that minimizes the adverse effects of gastrointestinal
reflux and c) an
excipient or combination of excipients thereof. In some embodiments, the excipient may increase interaction of the composition with the esophagus. Either short or
long acting acid inhibitors can be used in the dosage forms. Preferably the excipient allows convenient and efficient administration of the composition while increasing and maintaining interaction of the composition with the affected esophageal area for effective
therapeutic treatment.
[0015]Agents that enhance absorption of the composition through a surface of the gastrointestinal tract (e.g., the surface of the esophagus), may also be used to increase the interaction of the compositions disclosed herein with a surface of the gastrointestinal tract (e.g., the surface of the esophagus). Such agents include, but are not limited to, acylcarnitines, surfactants,
sodium lauryl
sulfate, saponins, bile salts or bile acids including but not limited to
cholanic acid, chilic acid,
deoxycholic acid,
glycocholic acid, tautocholic acid,
chenodeoxycholic acid,
lithocholic acid, ursocholic acid,
ursodeoxycholic acid, isourosde oxycholic acid, lagodeoxycholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, dehydrocholic acid,
hyocholic acid,
hyodeoxycholic acid, or combinations thereof, dihydrofusidates,
fatty acid derivatives,
chitosan, carbopol, cellulosic agents, sterols, including but not limited to alcohols structurally related to steroids, including but not limited to cholestanol, coprostanol,
cholesterol, epicholesterol,
ergosterol, ergocalciferol, or combinations thereof,
starch,
dextran,
cyclodextrin, or combinations thereof.
[0020]In a more general sense, the invention includes methods of treating esophageal inflammation, gastrointestinal reflux and / or other related conditions by orally administering a corticosteroid and one or more acid inhibitors at a
dose or
dose range effective to raise a patients
gastric pH to at least 3.5, preferably to at least 4 or and more preferably to at least 5, and to decrease the physiological manifestations, symptoms or appearance of esophageal inflammation, gastrointestinal reflux and / or other related
disease or ailment conditions. The patient is administered such compositions in one embodiment in a coordinated
dosage form with an excipient that increases interaction of the composition with the esophagus, including but not limited to
viscosity enhancing agents, mucoadhesive agents or absorption enhancers, or a combination thereof. In some aspects of the invention, a patient is administered with a single dosage unit form to achieve a
therapeutic effect. In other aspects, the patient may be administered the compositions disclosed herein for a single dose, or over a period of time, for example, at least two days, at least a week, at least a month or at least six months in order to achieve the desired
therapeutic effect. One of ordinary skill in the art would be knowledgeable as to the dosing amounts and
regimen of a patient to achieve a
therapeutic effect when treating with the compositions disclosed herein. For example, one of ordinary skill in the medical or pharmaceutical arts may monitor a patient's progress over time using physiological or
biochemical markers of esophageal inflammation and GERD further disclosed herein, and adjust or amend a dosing amount and / or
dosing regimen depending upon changes in the physiological or physical parameters of the patient to the compositions disclosed herein.
 Login to View More
Login to View More