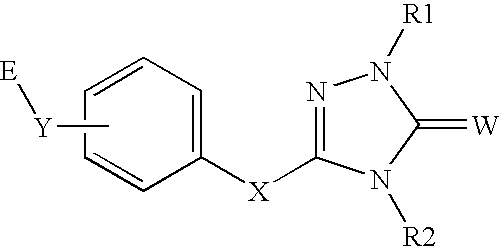
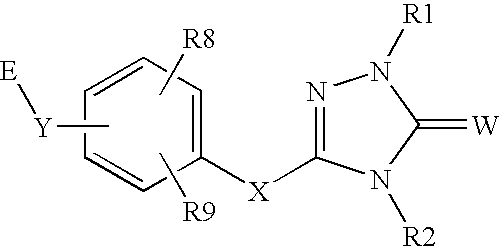
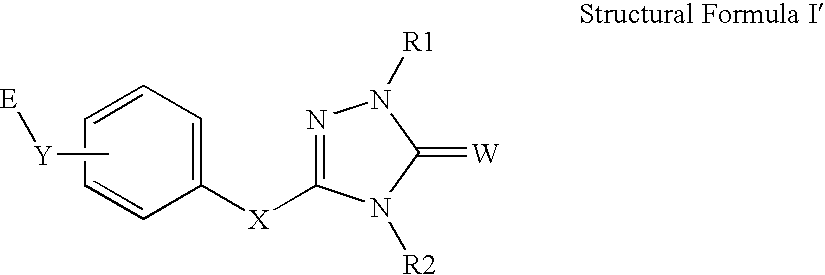
Peroxisome proliferator activated receptor alpha agonists
a technology of activated receptor and proliferator, which is applied in the field of peroxisome proliferator activated receptor alpha agonists, can solve the problems of liver toxicity, significant weight gain, tzds and ises typically have little effect, etc., and achieves fewer side effects, lowering fibrinogen, and increasing hdl levels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Compound 1(1)
[0206]Compound 1, shown below, was prepared according to the Steps outlined below:
Step A: Preparation of
[0207]
[0208]To a suspension of 4-(4-hydroxyphenyl)butyrylhydrazide (0.5 g, 2.58 mmol) in isopropanol (5 mL) was added 3-chloro-benzaldehyde (Aldrich, 420 mg, 3 mmol), followed by p-toluenesulphonic acid (25 mg). The reaction mixture was stirred at room temperature for 20 h. A solid separated, which was filtered, washed with isopropanol (0.25 mL), and dried to give the product as a solid. MS: m / z (M++1): 317 Additional compounds, shown below, were prepared by substituting the appropriate benzaldehyde for 3-chloro-benzaldehyde.
RMS: m / z (M+ + 1)3-Methylphenyl297Phenyl2832,4-Difluorophenyl3192-Methylphenyl2973-Methoxyphenyl313
Step B: Preparation of
[0209]
[0210]To a solution of the Step A product (650 mg, 2.05 mmol, Example 1) in a mixture of 5 mL of isopropanol, tetrahydrofuran, and acetic acid (1:1:0.3) was added sodium cyanoborohydride (1.25 g, 20 mmol). The reaction mix...
example 2
Compound 2(1)
[0223]The compound shown below was prepared as follows:
Step A: Preparation of:
[0224]
[0225]To a cooled (0° C.) solution of boron tribromide (50 g, 200 mmol) in CH2Cl2 (50 mL) was added a solution of methyl 4-(4-methoxyphenyl)butyrate (15.5 g, 74.4 mmol) in CH2Cl2 (100 mL) dropwise over one hour. After stirring for an additional hour at 0° C., the reaction mixture was treated with 1:1 CH3OH:CH2Cl2 (120 mL) with cooling and stirred overnight at ambient temperature. Concentration of the mixture gave an oil which was partitioned between ethyl acetate (150 mL) and water (150 mL). The aqueous layer was extracted with ethyl acetate (2×50 mL), and the combined organic extracts washed with water (50 mL), brine (50 mL), dried (Na2SO4), then concentrated to give the desired phenol as an oil. C11H14O3 (MW=194.23); MS: m / z (M++1)=195
Step B: Preparation of:
[0226]
[0227]The phenol from Step A (18.6 g, 96 mmol) was dissolved in DMF (300 mL) and treated with t-butyl 2-bromoisobutyrate (50...
example 3
[0239]
Step A: Preparation of:
[0240]
[0241]To a solution of the methyl ester, specifically Compound 2(1) (100 mg, 0.29 mmol) in DMF (2 mL), was added 3,4,5-trimethoxybenzyl chloride (129 mg, 0.6 mmol) and powdered potassium carbonate (350 mg, 2.53 mmol) and the resulting mixture heated at 45° C. for 24 hours. After cooling to ambient temperature, the reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (3×3 mL). The combined organic extracts were concentrated to an oil which was purified by flash chromatography (gradient elution, 1:4 ethyl acetate:hexanes to 4:1 ethyl acetate:hexanes) to give the desired product as an oil. C27H35N3O7 (MW=513.60); MS: m / z (M++1)=514
[0242]The compounds listed below were also prepared by alkylation of N-methyltriazolinone using appropriate alkylhalides in the place of 3,4,5-trimethoxybenzyl chloride.
RMS: m / z (M+ + 1)3,5-Dimethoxyphenyl4844-Biphenyl5003-Chlorophenyl4583-Chloro-4-methylphenyl472Bis-trifluoromethylphenyl5603,4-Di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com