Method of simultanaously detecting racemic methylephedrine hydrochloride, hydrobromic acid youmeishafen and clofenetamine maleate composition
A technology for racemic methylephedrine hydrochloride and chlorpheniramine maleate compositions, which is applied in the field of simultaneous detection of racemic methylephedrine hydrochloride, dextromethorphan hydrobromide and chlorpheniramine maleate compositions, It can solve the problems of poor precision, accuracy and reproducibility, not suitable for drug quality control, long equilibration time, etc., and achieve the effect of good reproducibility, strong practicability and good recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
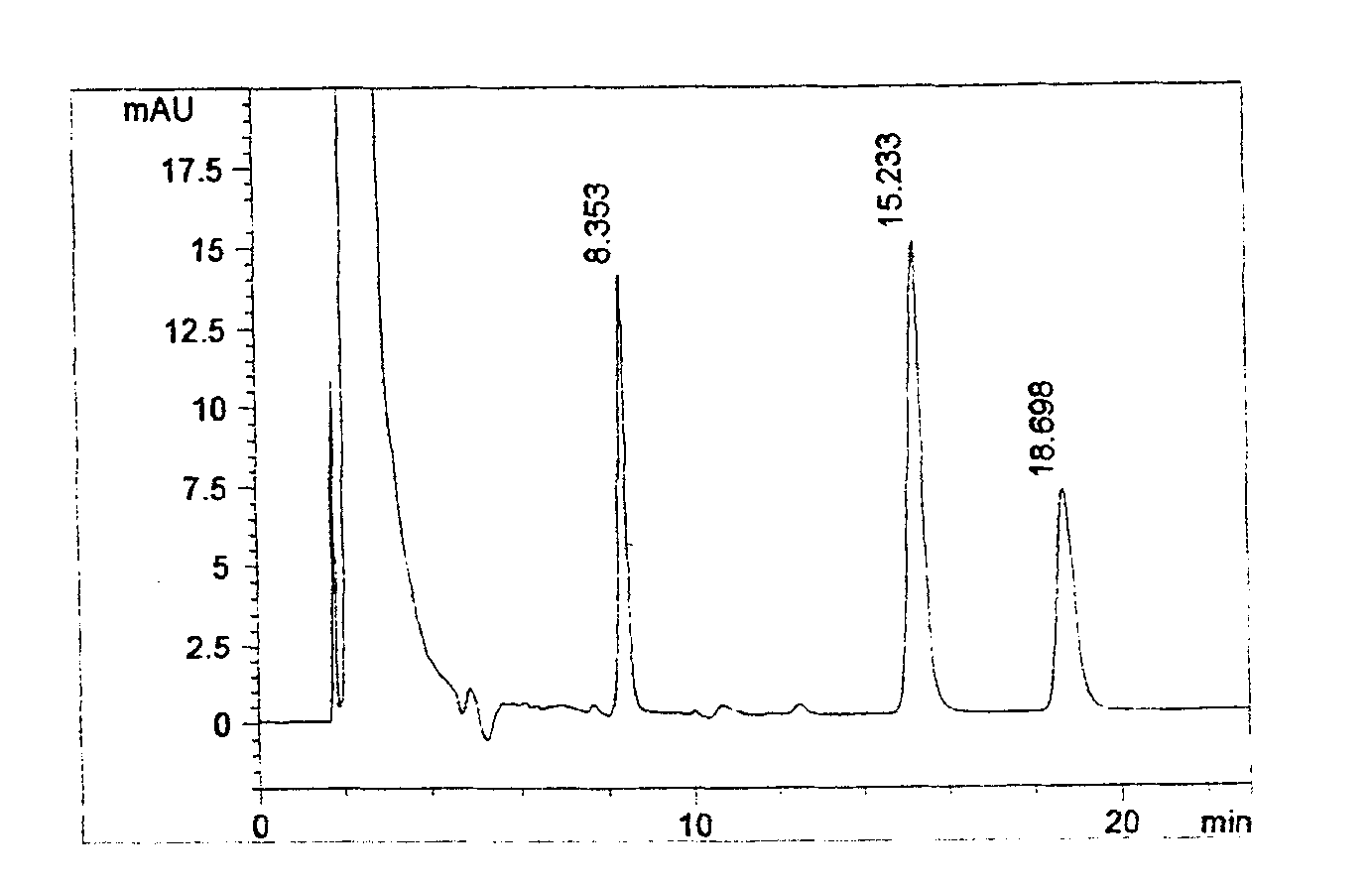
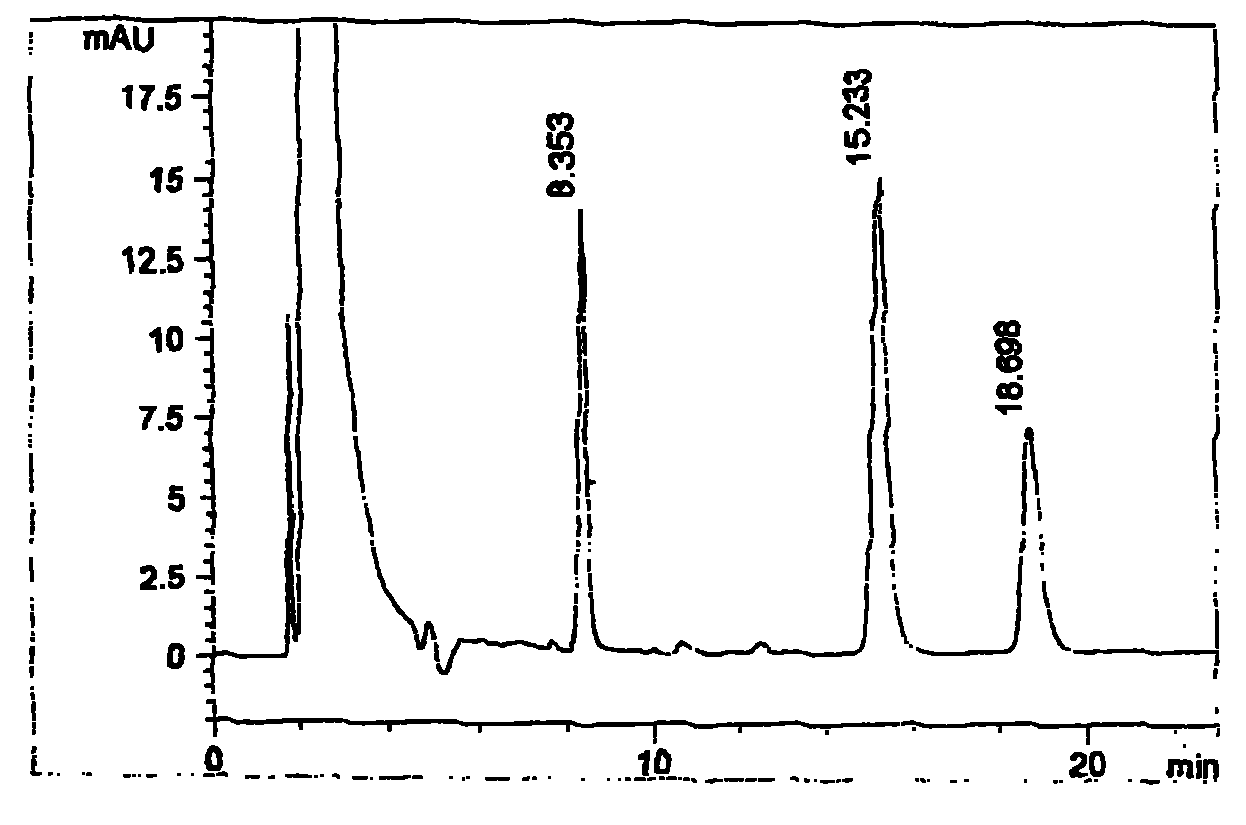
[0021] Use octadecylsilane bonded silica gel as filler; use methanol-acetonitrile-water-tetrahydrofuran (180:420:350:50), add 4.45g sodium dioctyl sulfosuccinate per 1000ml, adjust with phosphoric acid The pH value to 5.0 is the mobile phase, and the detection wavelength is 258nm. Take 15 grams of this product (containing about 50 mg of racemic methylephedrine hydrochloride, 40 mg of dextromethorphan hydrobromide, and 6.25 mg of chlorpheniramine maleate) in a 250 ml measuring bottle, add mobile phase for ultrasonic dissolution, and let cool until At room temperature, dilute to the mark with mobile phase, shake well, filter, accurately measure 20 μ l of the subsequent filtrate, inject it into the liquid chromatograph, and record the chromatogram; In the measuring bottle, add mobile phase to dissolve and dilute to the scale, as chlorpheniramine maleate reference substance stock solution; cancel methylephedrine hydrochloride reference substance 20mg, dextromethorphan hydrobromide...
Embodiment 2
[0023] Use octadecylsilane bonded silica gel as filler; use ethanol-acetonitrile-water-tetrahydrofuran (150:450:370:30), add 5.0 g of sodium dioctyl sulfosuccinate per 1000 ml, adjust with acetic acid The pH value to 5.0 is the mobile phase, and the detection wavelength is 258nm. Take 15 grams of this product (containing about 50 mg of racemic methylephedrine hydrochloride, 40 mg of dextromethorphan hydrobromide, and 6.25 mg of chlorpheniramine maleate) in a 250 ml measuring bottle, add mobile phase for ultrasonic dissolution, and let cool until At room temperature, dilute to the mark with mobile phase, shake well, filter, accurately measure 20 μ l of the subsequent filtrate, inject it into the liquid chromatograph, and record the chromatogram; In the measuring bottle, add mobile phase to dissolve and dilute to the scale, as chlorpheniramine maleate reference substance stock solution; cancel methylephedrine hydrochloride reference substance 20mg, dextromethorphan hydrobromide ...
Embodiment 3
[0025] Use octadecylsilane bonded silica gel as a filler; use methanol-acetonitrile-water-tetrahydrofuran (200:400:330:70), add 3.5g sodium dioctyl sulfosuccinate per 1000ml, adjust with phosphoric acid The pH value to 5.0 is the mobile phase, and the detection wavelength is 258nm. Take 15 grams of this product (containing about 50 mg of racemic methylephedrine hydrochloride, 40 mg of dextromethorphan hydrobromide, and 6.25 mg of chlorpheniramine maleate) in a 250 ml measuring bottle, add mobile phase for ultrasonic dissolution, and let cool until At room temperature, dilute to the mark with mobile phase, shake well, filter, accurately measure 20 μ l of the subsequent filtrate, inject it into the liquid chromatograph, and record the chromatogram; In the measuring bottle, add mobile phase to dissolve and dilute to the scale, as chlorpheniramine maleate reference substance stock solution; cancel methylephedrine hydrochloride reference substance 20mg, dextromethorphan hydrobromid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com