Fluoroboric dye fluorescent probe for cell zinc ion detection
A technology of fluorescent probes and zinc ions, which is applied in the direction of luminescent materials, biological testing, material inspection products, etc., can solve the problems of difficult synthesis of probe molecules, complex structures, difficulties, etc., and achieve high yield, no toxic side effects, and stable good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Probe 3
[0032]
[0033] (1) Synthesis of Intermediate 2
[0034] In a 1000ml three-necked flask, add 2.17 g (19.4 mmol) of chloroacetyl chloride and 3.7 g of 1,4-dimethylpyrrole to dissolve in 2 L of dichloromethane, stir at room temperature for 5 hours under nitrogen protection, and evaporate the mixed solution to dryness under reduced pressure. 0.5L, then 40ml of triethylamine was added, and then 80ml of boron trifluoride was added, and the mixture was continuously stirred for 4 hours. The mixture was washed with water, dried and evaporated to dryness. Silica gel column separation (16:1 n-hexane:ethyl acetate) afforded 1.69 g of Intermediate 2. Yield 34%. 1 H-NMR (400MHz, CDCl 3 , ppm): δ2.53 (s, 12H); 4.78 (s, 2H); 6.08 (s, 2H). 13 C-NMR (400MHz, CDCl 3 , ppm): δ14.86, 15.69, 37.33, 122.46, 131.57, 136.14, 141.31, 156.83. 19 F(400MHz, CDCl 3 , CFCl 3 asexternal reference, δ=0): δ-146.73(q, J=36Hz). Anal.Calcd for C 14 H 16 BClF 2 N 2 :...
Embodiment 2
[0038] Probe 3 zinc ion selectivity
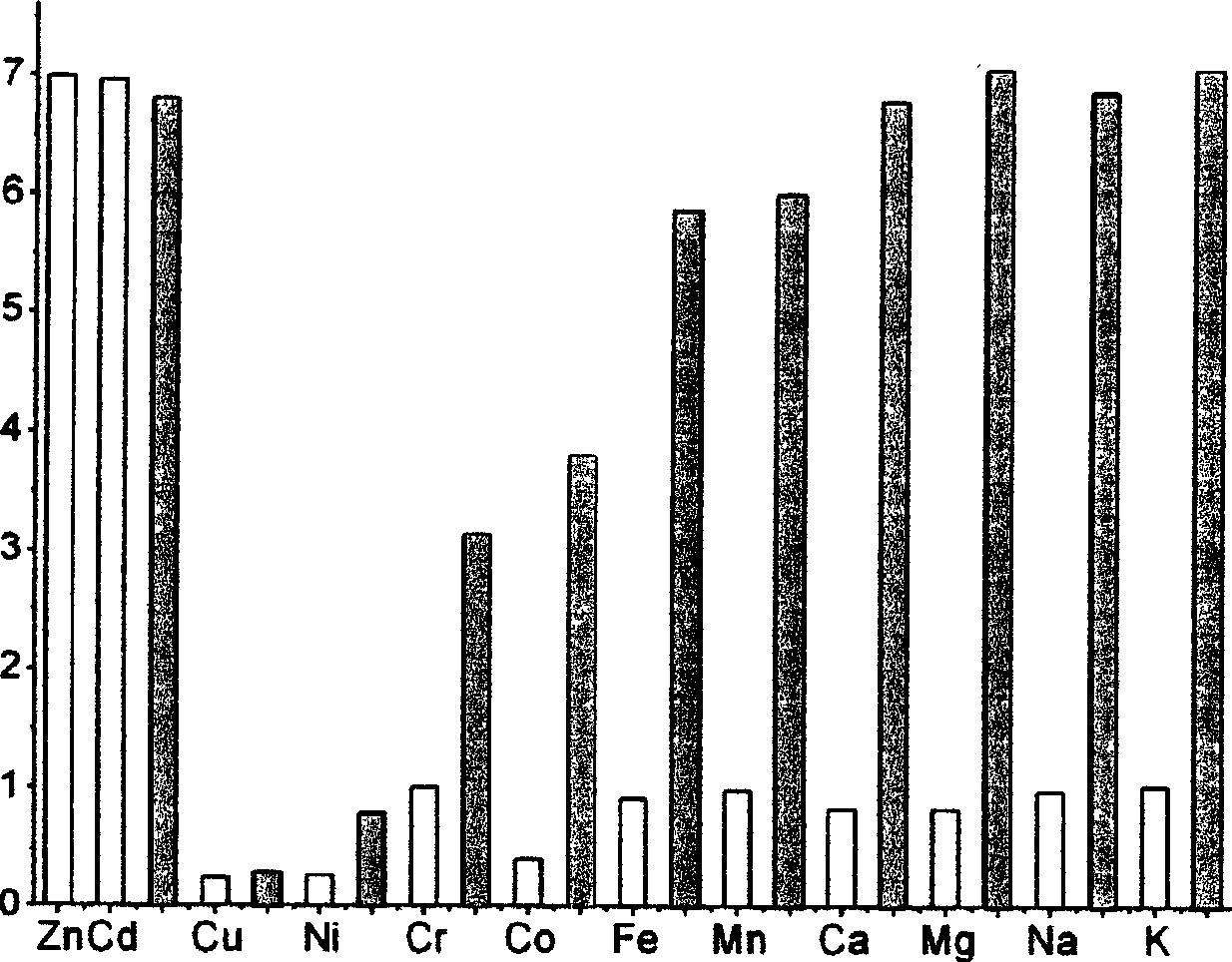
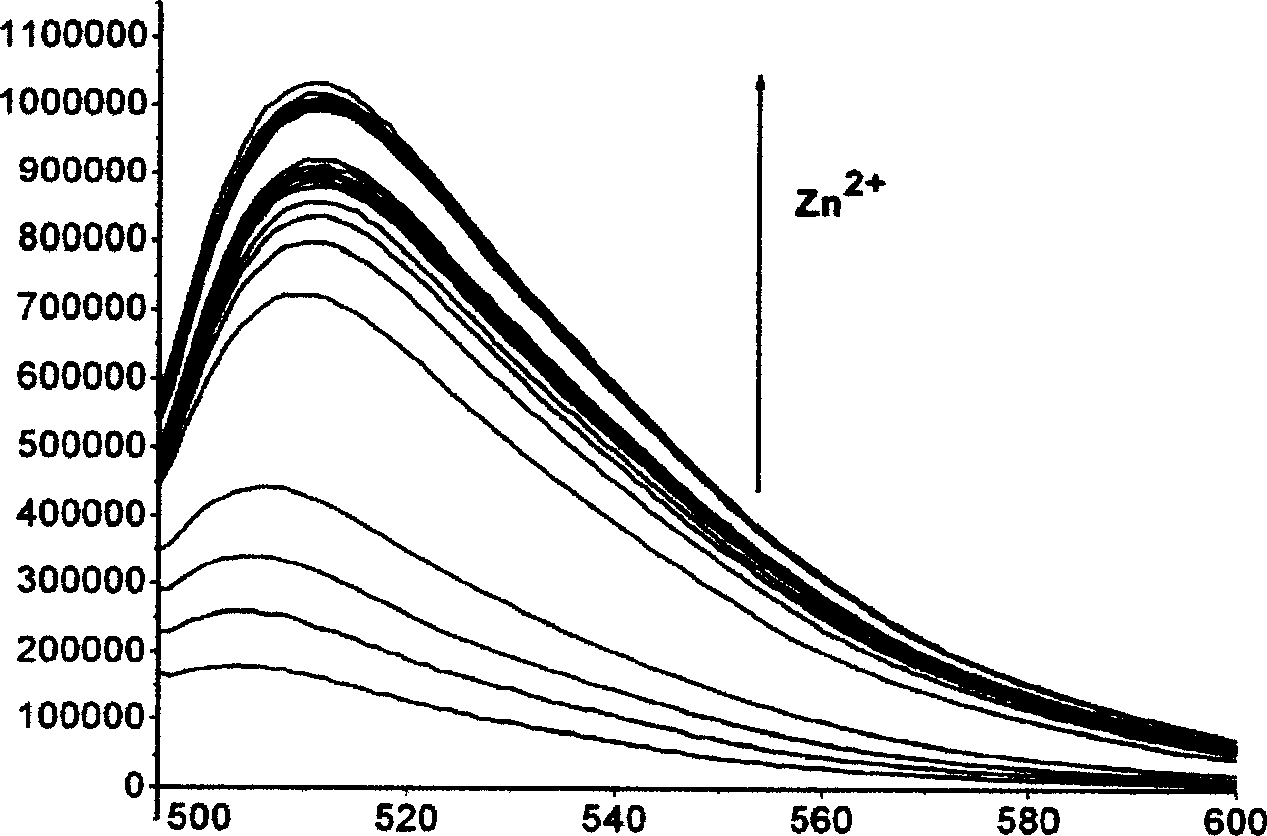
[0039] The selectivity to zinc ions was evaluated using compound 3 synthesized above. 1 μM of compound 3 was added to a five-fold excess of various metal ions in Tris-HCl buffer at pH 7.4 (20 mM). The excitation wavelength of probe 3 was 492 nm and the emission wavelength was 511 nm. The test results are shown in In Figure 1 . In the figure, the fluorescence intensity on the vertical axis is set to 1 when the metal ion is not added, and the fluorescence intensity when various metal ions are added is represented by numerical values. It can be seen from the figure that compound 3 has high sensitivity to zinc ions, the addition of zinc ions produces a great fluorescence enhancement, and also has good selectivity to zinc ions, such as sodium, potassium, calcium, magnesium, and manganese. , iron and other metal ions do not interfere with the detection.
Embodiment 3
[0041] Insensitivity of probe 3 to pH
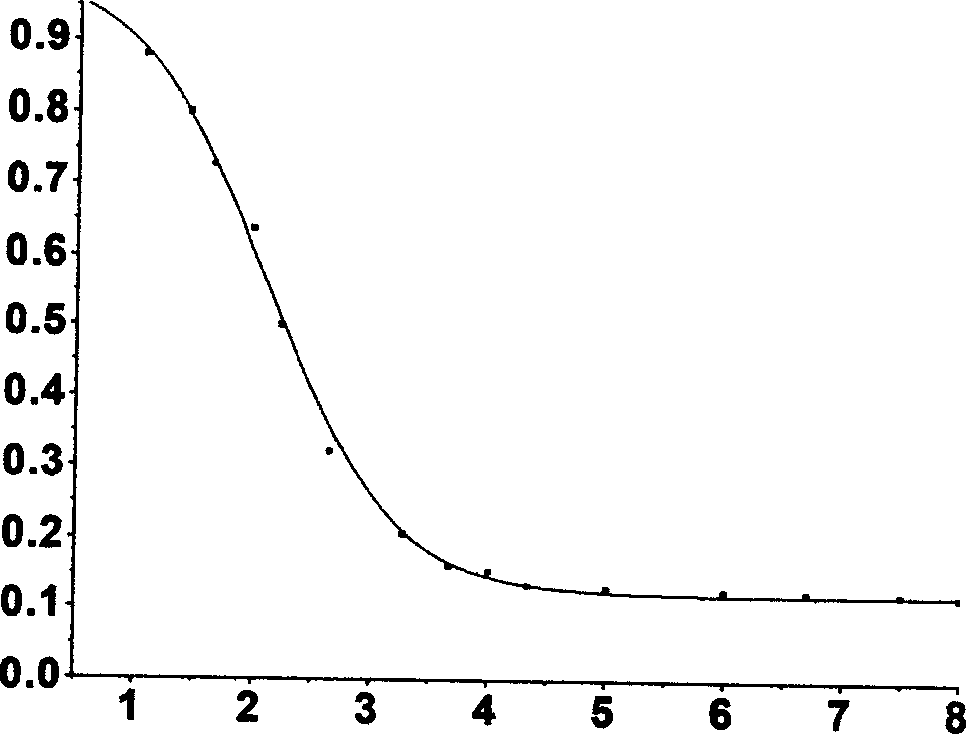
[0042] Use the compound 3 synthesized above to evaluate the response to pH. For compound 3, adjust the pH value to about 1.0 in an aqueous solution with an ionic strength of 0.1. After measuring the fluorescence intensity, add lye to slowly increase the pH value and record the corresponding fluorescence. Intensity changes, test results are shown in In Figure 3 . It can be seen from the figure that the probe 3 has a very low pKa value, and in the pH range of 4 to 10, the pH change has little effect on the fluorescence emission. In addition, the same method was used to measure the pH response of 1:1 zinc ion complex with compound 3. The test results are shown in Figure 3 in the illustration. It can be seen from the figure that in the range of pH 3 to 10, the pH change has little effect on the fluorescence emission. Therefore, probe 3 can be used for the detection of intracellular zinc ions in a wide pH range.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com