Quality controlling method for dangshen and preparation containing it
A quality control method and control method technology, applied in the field of quality control of Codonopsis pilosula and preparations containing Codonopsis pilosula, can solve the problems of ineffective quality control of medicinal materials and pharmaceutical preparations, insufficient research on active ingredients of Codonopsis pilosula, etc., and achieve accurate quantitative determination, The effect of accurate evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
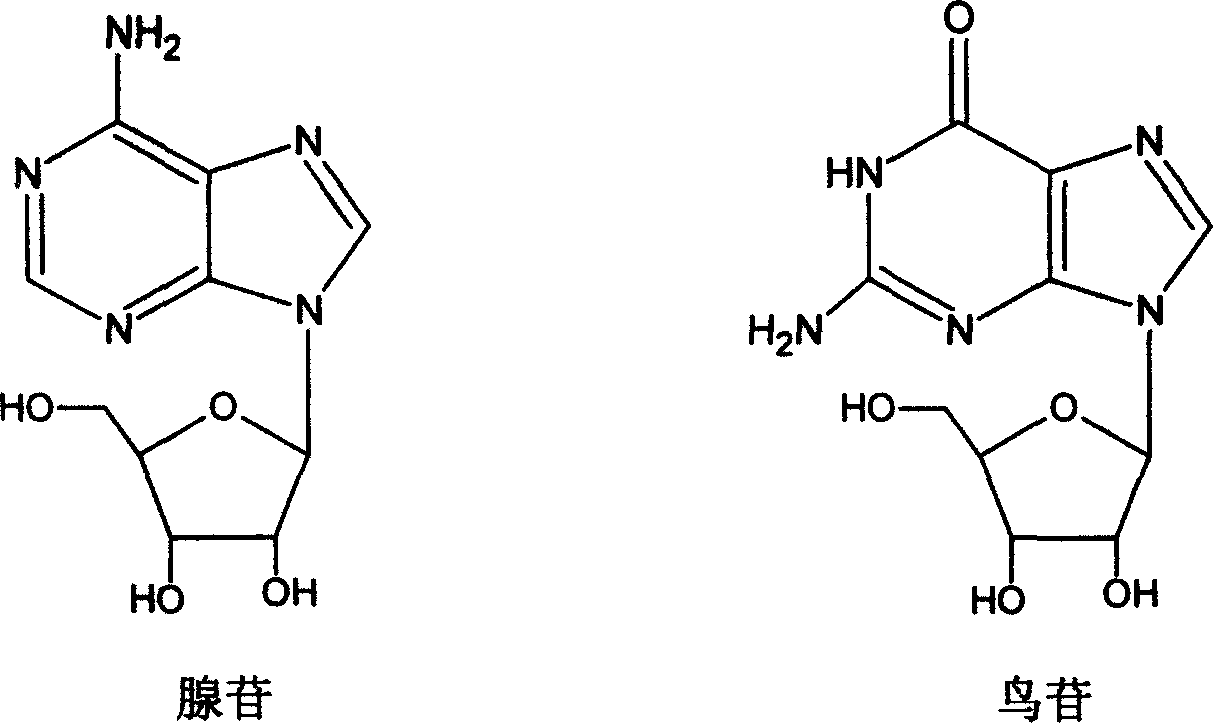
[0013] Example 1: Determination of the Content of Adenosine and Guanosine in Codonopsis Codonopsis Medicinal Materials by High Performance Liquid Chromatography
[0014] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler; column temperature is 25°C, detection wavelength is 260nm. Mobile phase A is water+glacial acetic acid (970ml+1ml), mobile phase B is acetonitrile, gradient elution: start with 3% mobile phase B (that is, mobile phase A is 97%), maintain isocratic elution for 8 minutes, and then Increase the proportion of mobile phase B at a rate of 6.7% per minute to 50% B after 15 minutes, hold for another 5 minutes, and end the gradient at 20 minutes. The flow rate was 1.0 ml / min. The injection volume is 20 μl, and the number of theoretical plates should not be less than 3000 based on adenosine, and not less than 3000 based on guanosine.
[0015] Preparation of the test solution: Grind Codonopsis pilosula, weigh 1g of t...
Embodiment 2
[0020] Example 2: Determination of the content of adenosine and guanosine in Codonopsis Codonopsis medicinal materials by high-efficiency thin-layer chromatography scanning method
[0021] Thin-layer chromatography conditions Use a fully automatic spotting instrument to spot the sample in strips on a silica gel GF254 thin-layer plate, and place it in a developing cylinder for development. Developing agent: chloroform-methanol-n-butanol-ethyl acetate-concentrated ammonia water (9 :4:3:3:1), unfolded 9cm, took out to dry, observed under ultraviolet light with a wavelength of 254nm, and scanned and integrated at a wavelength of 270nm with a thin-layer scanner.
[0022] Preparation of test solution: Pulverize Codonopsis pilosula, weigh 5g of powder (through No. 2 sieve), accurately weigh, place in a stoppered Erlenmeyer flask, add 50ml of 50% methanol, soak at 70°C for 3 hours, filter, The filtrate was concentrated to dryness at 70°C under reduced pressure, dissolved in 10ml of wa...
Embodiment 3
[0027] Example 3: Determination of the Content of Adenosine and Guanosine in Compound Dangshen Tablets by High Performance Capillary Electrophoresis
[0028] Capillary electrophoresis conditions Running buffer: 50mmol / L borax solution plus 18% methanol (pH9.7); pressure injection: 5kPa, 4s; separation voltage: 24kV: temperature of capillary and automatic sampling tray: 25°C; detection wavelength: 254nm . Before use, the capillary column was washed with 1mol / L sodium hydroxide solution, deionized water and running buffer for 10 minutes each, and washed with running buffer for 10 minutes after each electrophoresis.
[0029] Preparation of the test solution: Peel off the sugar-coated tablets of the compound Codonopsis pilosula and pulverize them, weigh 1 g of the powder (passed through a No. 2 sieve), place it in a stoppered Erlenmeyer flask, add 20 ml of 70% methanol, soak at 70 ° C for 2 hours, Take it out, place it at room temperature for 1.5 hours, filter, and take the conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com