Synthesis method of halogenated biaryl compound
A compound, biaryl technology, applied in the field of synthesis of halogenated biaryl compounds, can solve the problems of harsh reaction conditions, poor selectivity, low yield, etc., to achieve reduced side reactions, high yield, universal substrate sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention provides a preparation method of a halogenated biaryl compound, which comprises the following steps:
[0046] Under non-reactive gas atmosphere, dihalogenated aryl compound, aryl Grignard reagent, [1,3-bis(2,6-diisopropylbenzene)imidazole-2-idene](3-chloropyridine) Palladium dichloride, alkali and solvent are mixed, and then reacted at 50°C to 75°C;
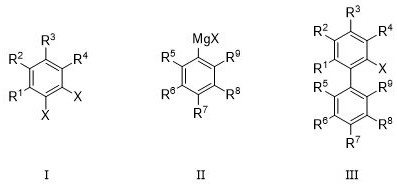
[0047] The dihalogenated aryl compound has the structure shown in formula I, the aryl Grignard reagent has the structure shown in formula II, and the halogenated biaryl compound has the structure shown in formula III:
[0048]
[0049] wherein each occurrence of X is independently selected from -Br or -I;
[0050] R 1 ~R 9 Each occurrence is independently selected from -H, -D, -NMe 2 , -NO 2 , -CF 3 , halogen, unsubstituted or at least one R 10 substituted C 1 ~C 10 Alkyl or alkoxy, unsubstituted or at least one R 11 substituted C 3 ~C 6 Cycloalkyl, unsubstituted or at least one R 12 substitut...
Embodiment 1
[0081] Under Ar atmosphere, 1,4-dimethoxy-2,3-dibromobenzene (No. 1-4, 30 g, 1.0 eq), Pd-PEPPSI-IPr catalyst (1.38 g, 2 mol%) and t BuONa (14.61 g, 1.5 eq) was stirred at room temperature for 30 min; then 2,4,6-triisopropylbenzenemagnesium bromide Grignard reagent (No. II-8, 1.3 eq) was slowly added, the temperature was raised to 70 °C, TLC monitoring When the reaction is complete, the reaction is stopped and cooled to room temperature;
[0082] 500 mL of water was slowly added to the reaction system, the layers were separated and the organic phase was retained. The aqueous phase was extracted with ethyl acetate (300 mL×3). All organic phases were combined and washed with saturated sodium chloride solution (1 L×1). Dry with sodium sulfate, filter, remove the solvent by distillation under reduced pressure, and purify by column chromatography to obtain the target product No. III-12, which is 37.8 g of white solids, and the yield is 89%.
[0083] III-12 NMR data: 1 H NMR (400 ...
Embodiment 2~20
[0085]Basically the same as Example 1, the difference is that some reaction conditions are different, for example, different catalyst types, different catalyst amounts, different amounts of aryl Grignard reagents, different types of bases, different types of solvents or different reaction temperatures are used. Table 1:
[0086] Table 1
[0087]
[0088] Analyze the data of table 1, as can be known from embodiment 1~5, the selection of Pd-PEPPSI-IPr catalyst has a decisive influence on the smooth occurrence of realization reaction, other common palladium catalyst, or can not react at all (such as embodiment 4) , or the yield is very low. It can be seen from Examples 1, 6 to 9 that with the increase of the catalyst dosage, the reaction yield will also increase, but the increase in the yield becomes very limited when it increases to 3%. It can be seen from Examples 1 and 10-12 that the amount of aryl Grignard reagent will also affect the reaction yield. As the amount increa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com