Asymmetric phosphorus oxide pyridine triazine derivative and synthesis method thereof
A technology of phosphorus oxide pyridine triazines and synthetic methods, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., capable of solving trivalent minor actinide ions and lanthanide ions Separated from each other, etc., to achieve good application prospects, strong extraction ability, and the best effect of extraction ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
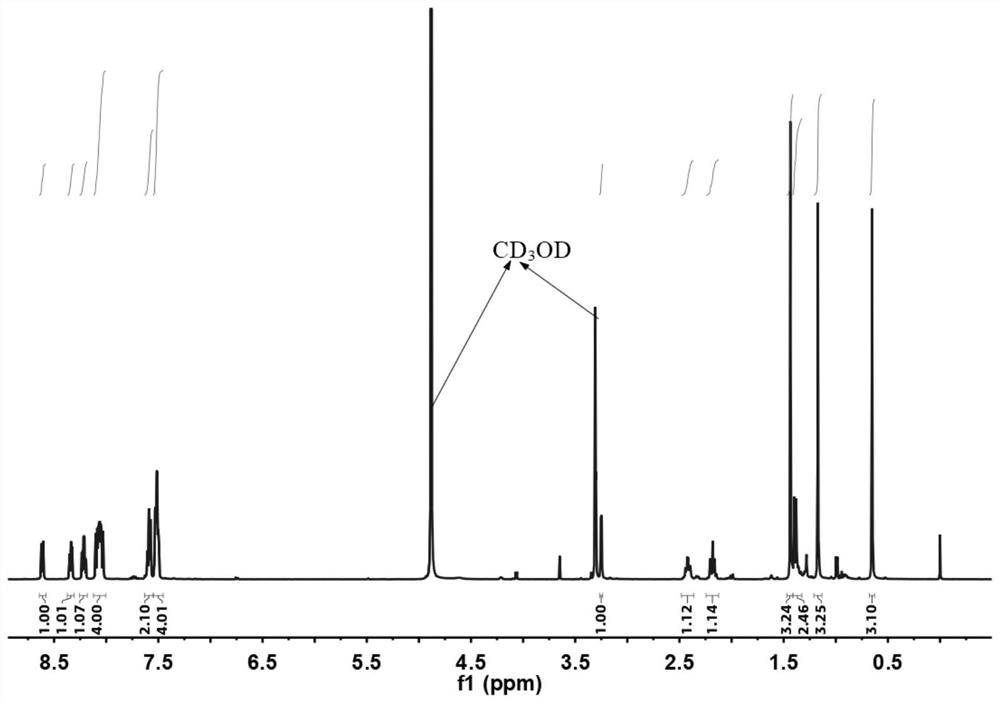
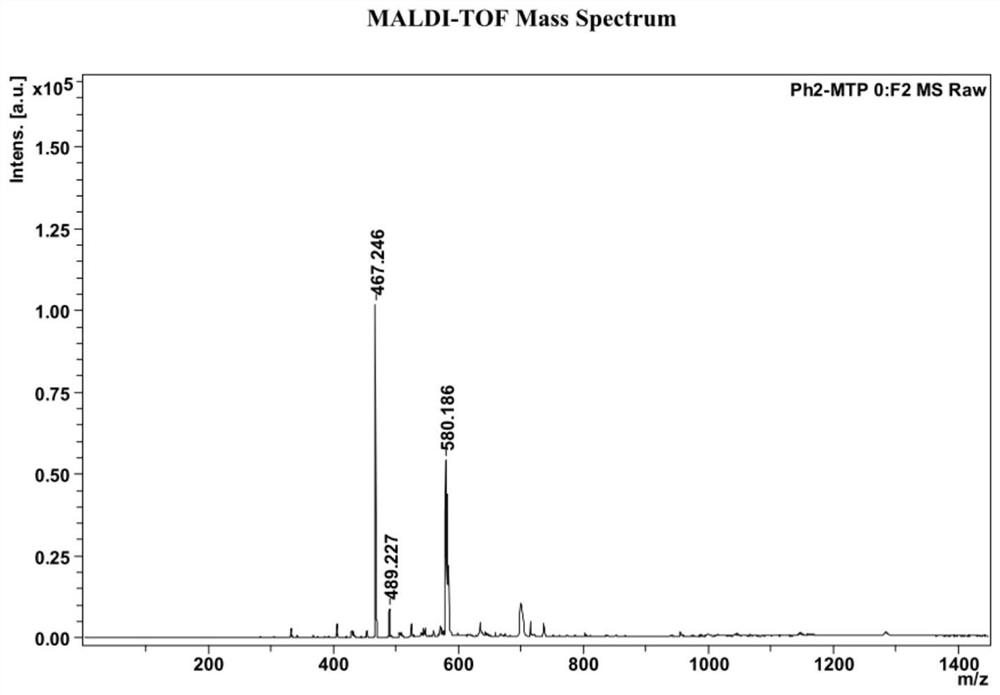
[0044] Example 1 Ph 2 Synthesis of CA-TPPO
[0045] Step 1: Put 2.5g of 6-bromo-2-cyanopyridine and 10.0ml of hydrazine monohydrate into a reaction flask, add 10.0mL of deionized water and react at 25°C for 1.0h. After the reaction, the solid powder was filtered directly, washed three times with deionized water, and then dried overnight at 60° C. to obtain intermediate product 2, about 2.0 g.
[0046]
[0047] Step 2, 2.3g of camphorquinone is dissolved in ultra-dry ethanol or tetrahydrofuran, and is added dropwise to the ultra-dry ethanol suspension solution containing 2.0g of brominated monopyridylamine hydrazone (intermediate product 2 prepared in step 1) through a constant pressure funnel, , and react after the dropwise addition is completed. The reaction was first refluxed for 3 h under the protection of high-purity argon, and then stirred overnight at room temperature. The reaction was tracked by TLC until the reaction of the raw materials was complete.
[0048] Obta...
Embodiment 2
[0059] Synthesis of Example 2 BuPhCA-TPPO
[0060] Take 1g of the intermediate product 4 prepared in step 2 of Example 1, dissolve it with 0.8g butylphenylphosphine oxide in 50.0mL ultra-dry toluene solution and add 28.0mg (0.122mmol) of palladium acetate to the solution, dppf 1, 160.0mg (0.244mmol) of 1'-bis(diphenylphosphino)ferrocene and 3.0g of cesium carbonate were vacuumized under the protection of argon, and refluxed at 110°C for 24h. After the reaction was completed, the solvent toluene was removed by suction filtration and rotary evaporator. Dichloromethane and saturated NaCl solution were added to the crude product, and the organic phase was combined after washing and extraction several times to obtain a brown oil, which was treated with dichloromethane / ethyl acetate / methanol (v / v / v=30:30:1) After separation by column chromatography as an eluent, 0.8 g of the product BuPhCA-TPPO brown oil was obtained.
[0061]
[0062] (1) BuPhCA-TPPO 1 H NMR characterization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com